Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The emergence of biologic therapies targeting eosinophils via the IL-5 pathway provided new options for treatment in eosinophilic granulomatosis with polyangiitis (EGPA). There has also been an increase in biologic therapies targeting other pathways for severe asthma, a prominent feature of EGPA. This study evaluated the evolution of systemic therapies utilized in patients with EGPA before and after introduction of these biologic agents in North America.
Methods: Clinical data were examined from 326 participants in a longitudinal cohort at 11 academic centers in the USA and Canada. Participants were grouped based on the timing of their enrollment in the cohort, using three separate periods: (A) April 2006 — April 2011; (B) May 2011 (first full month after FDA approval of rituximab for use in other forms of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis) — December 2017; (C) January 2018 (first full month after FDA approval of mepolizumab for use in EGPA) — October 2023.
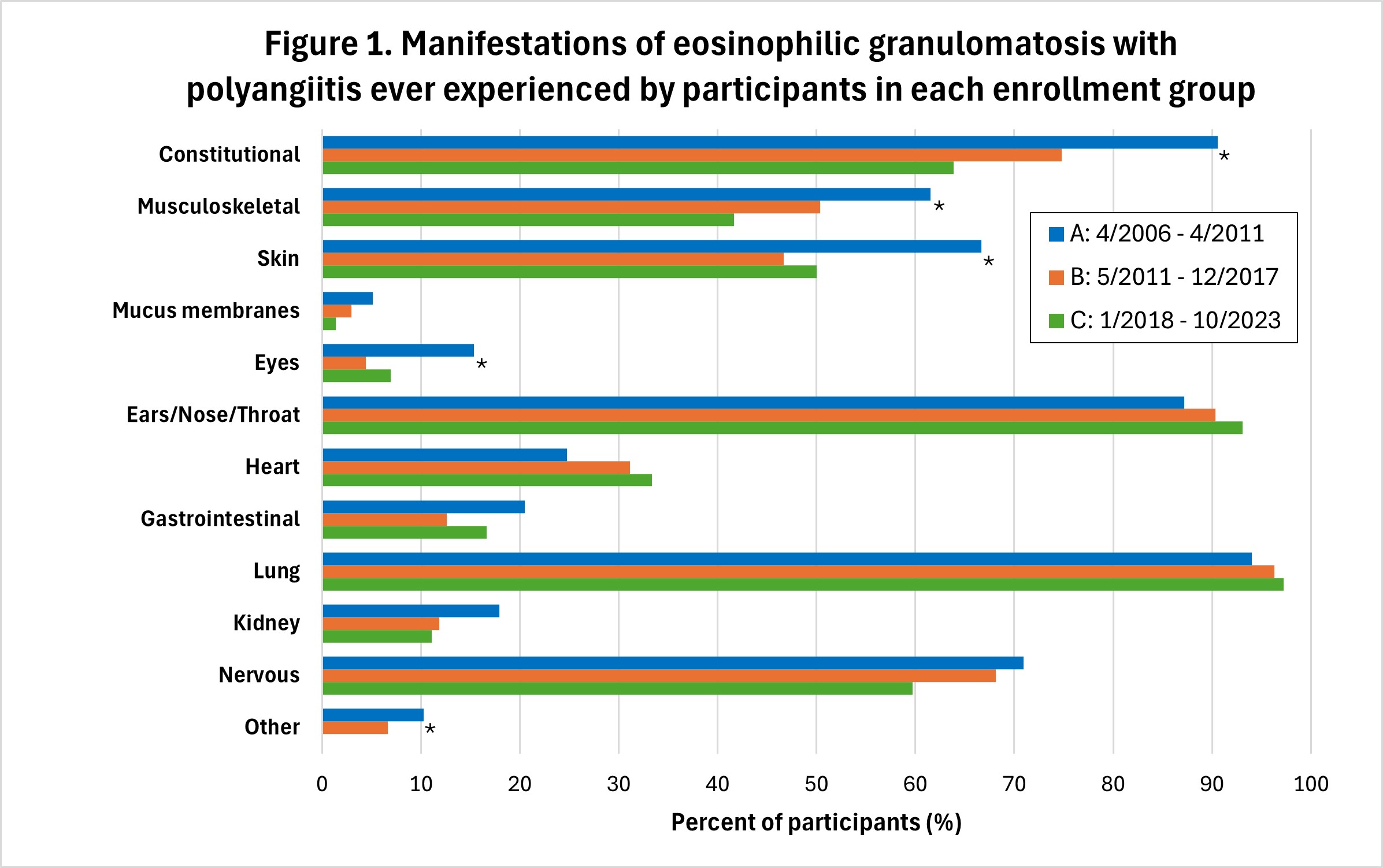
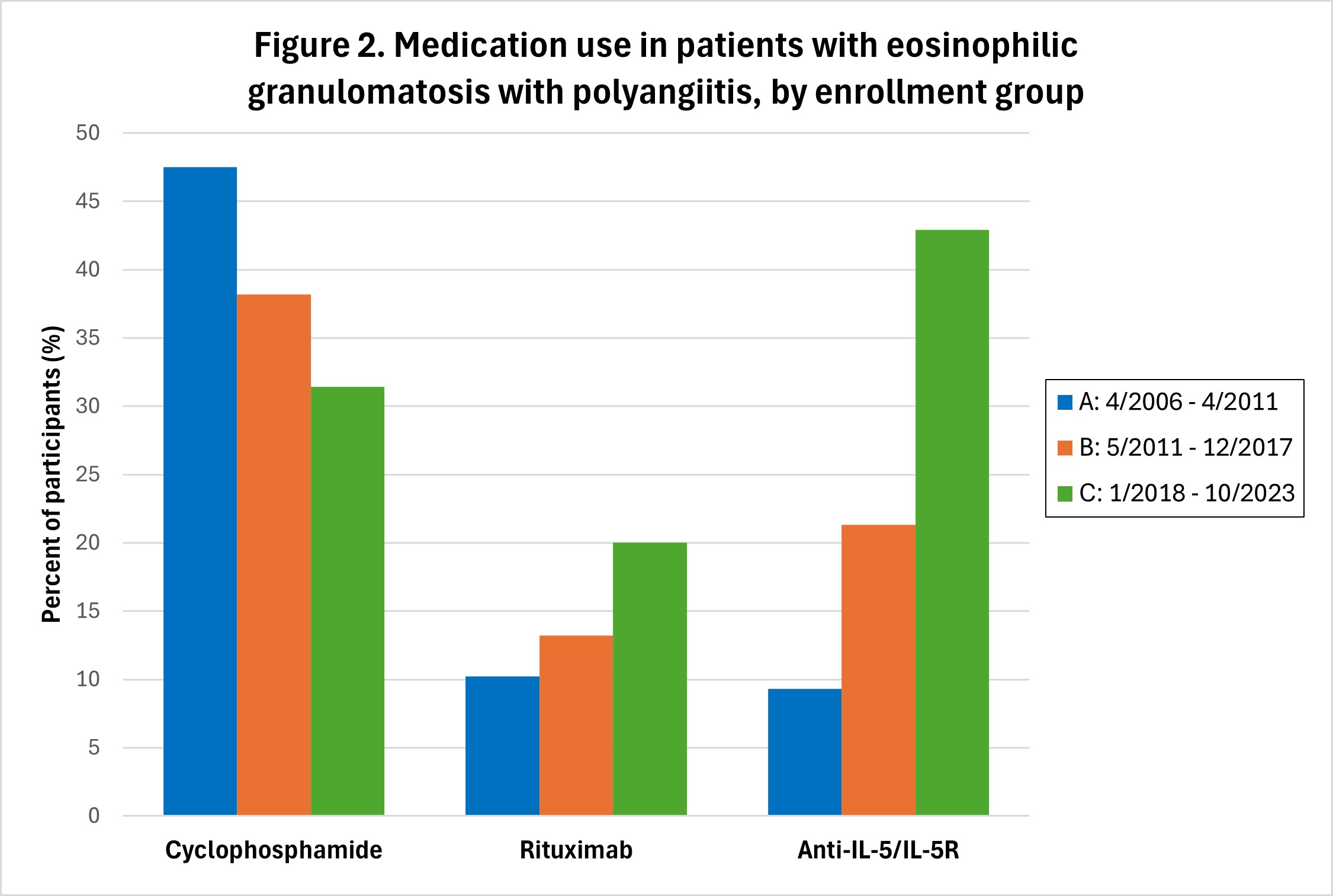
Results: Enrollment per period: A-118 participants; B-136; C-72. Sex and age distribution were similar across all time periods, with 187 (57.4%) females, and mean ± SD age at enrollment = 53.4 ± 14.2 years. Median disease duration at enrollment = 1.5 years; 127/326 (40.0%) were ANCA-positive. Manifestations of EGPA experienced by the participants at any point during their disease course were generally similar across the three enrollment periods (Figure 1). Cyclophosphamide (IV or oral) was used during the treatment course of 56 (47.5%) participants enrolled during A, 52 (38.2%) during B, and 22 (31.4%) during C (p=0.0792) (Figure 2). Rituximab was used in 12 (10.2%) patients enrolled in period A, 18 (13.2%) during B, and 14 (20.0%) during C (p=0.1762). 70 (21.6%) participants received an anti-IL-5 or anti-IL5R agent (mepolizumab, benralizumab, or reslizumab) during their course, most commonly mepolizumab (n=53). Use of these three agents increased over time, with 11 (9.3%) participants enrolled in period A ultimately receiving one of these agents, versus 29 (21.3%) in group B, and 30 (42.9%) in group C (p< 0.0001). Two participants received concurrent treatment with mepolizumab and rituximab (1 each in groups B and C). One participant, enrolled during period B, received concurrent cyclophosphamide and mepolizumab. 14 participants (4.3%) received a non-IL5/IL5R biologic asthma therapy (omalizumab or dupilumab; none with tezepelumab), of whom 12 started prior to enrollment in the study cohort (one received both prior). Participants receiving omalizumab were distributed across all 3 enrollment groups (A=4, B=5, C=2), while all 4 participants receiving dupilumab were in enrollment group C and started this drug prior to enrollment.
Conclusion: Treatment regimens for EGPA evolved substantially over time, with changes in prescribing patterns closely linked to availability of biologic immunomodulatory agents. Further analyses are warranted to investigate the efficacy and safety of these agents for management of specific disease features of EGPA.
To cite this abstract in AMA style:
Fussner L, Pagnoux C, Cuthbertson D, Corbridge T, Khalidi N, Koening C, Langford C, McAlear C, Monach P, Moreland L, Rhee R, Seo P, Shavit A, Silver J, Specks U, Warrington K, Wechsler M, Merkel P. Changing Spectrum of Systemic Therapies for Eosinophilic Granulomatosis with Polyangiitis from 2006-2023 [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/changing-spectrum-of-systemic-therapies-for-eosinophilic-granulomatosis-with-polyangiitis-from-2006-2023/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/changing-spectrum-of-systemic-therapies-for-eosinophilic-granulomatosis-with-polyangiitis-from-2006-2023/