Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Although methotrexate (MTX) is a first-line treatment for inflammatory rheumatic diseases, side effects can lead to poor adherence and persistence. A novel intervention involves targeting mindsets about non-severe medication side effects, re-framing them as encouraging signs from the body that medication is working. The objective of this randomized controlled trial (RCT) was to assess whether a brief symptom-mindset intervention can improve side effect experience and adherence in patients starting MTX.
Methods: People with inflammatory rheumatic diseases starting MTX were recruited into a single-blind RCT. Participants were randomized (1:1) to a mindset intervention group or standard information control group. Both groups were shown a video about MTX distinguishing between severe and non-severe symptoms. The mindset intervention video (7:15min) framed non-severe symptoms as positive signs of MTX’s activity. The standard information video (5:00min) matched clinic messaging, framing non-severe symptoms as side effects. Participants were blinded to the study hypothesis. The primary endpoint was symptom experiences at 4-weeks. Symptom mindset and burden were assessed when symptoms were reported, and perceived MTX effectiveness was assessed when no symptoms were reported. Adherence was also assessed after 4 weeks, along with motivation to use MTX compared to baseline. Continuation of MTX was assessed after 12 weeks. Chi-square tests of independence were conducted on categorical outcomes. Independent samples t-tests and mixed-model ANOVA were used to test single- and repeated-measure continuous outcomes, respectively.
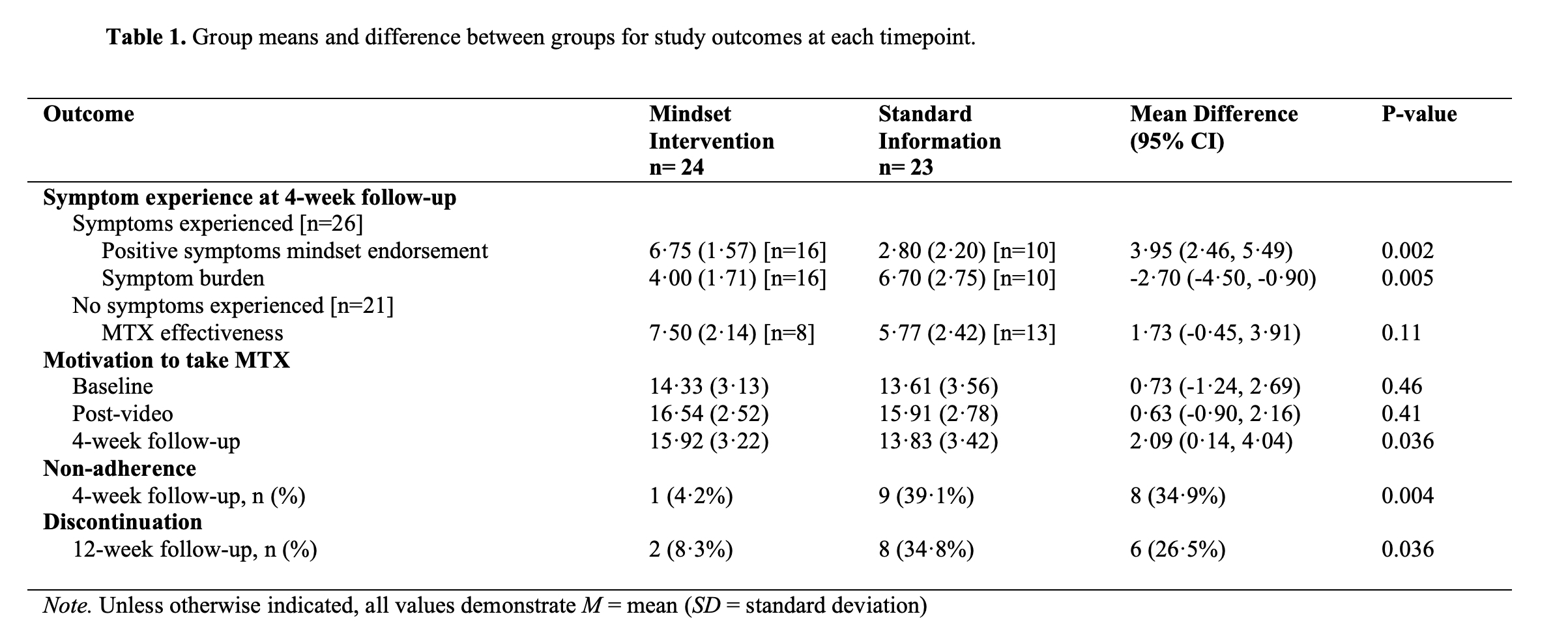
Results: All 47 participants randomized to the intervention (n=24) or control group (n=23) completed the study. Most participants were female (74%) with a mean [SD] age of 53.6 [15.7]. Rheumatoid arthritis was the most common diagnosis (42%). Study outcomes are shown in Table 1. Amongst the 26 participants who experienced symptoms after a MTX dose, the intervention group had greater endorsement of the mindset that these symptoms were positive signals and experienced them as less burdensome than the control group (mean difference on a 0-10 Likert scale -2·70 (95% CI -4·50 to -0·90), P=.005). Amongst participants who did not experience symptoms, there was no difference in perceived effectiveness of MTX. The intervention group reported better adherence compared to the control group after 4-weeks. Both groups had higher motivation to take MTX after seeing the videos, with greater motivation in the intervention group at 4-weeks. After 12-weeks, the intervention group had a better MTX continuation rate than the control group.
Conclusion: In patients starting MTX, a mindset intervention that re-frames the role of non-severe side effects is a promising approach for improving symptom experience and early-stage medication persistence.
To cite this abstract in AMA style:
Yielder R, Leibowitz K, Crum A, Manley P, Dalbeth N, Petrie K. Changing Patients’ Mindsets About Non-Severe Side Effects of Methotrexate: A Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/changing-patients-mindsets-about-non-severe-side-effects-of-methotrexate-a-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/changing-patients-mindsets-about-non-severe-side-effects-of-methotrexate-a-randomized-controlled-trial/