Session Information
Date: Friday, November 6, 2020
Title: Patient Outcomes, Preferences, & Attitudes Poster I: RA, Spondyloarthritis, & OA
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Methotrexate (MTX) is frequently used in patients with rheumatoid arthritis (RA) or psoriatic arthritis (PsA) because of its beneficial effects in both populations. Despite the well-known benefits of MTX, it is associated with a number of potential side effects. These include nausea and fatigue, are often temporally related to the timing of weekly MTX administration, and can be severe. The combined patient-reported side effects, along with potential of long term toxicity, may make use of MTX more burdensome. Currently, there is a gap in patient-centered studies that focus on patients’ experience of fatigue and nausea relating to oral MTX therapy for the treatment of RA and PsA.
Methods: Adult US patients in the ArthritisPower registry with self-reported RA or PsA taking MTX for less than 10 years were invited to participate in the study via email invitation. Participants (pts) completed a screener and brief online survey. In an ancillary study to the ArthritisPower registry and using a self-controlled case series study design where pts serve as their own control to avoid between-person confounding, pts were asked to complete a set of up to 8 assessments within 6-36 hours (‘risk’) and 96-144 hours (‘control’) after taking their oral dose of MTX each week, for up to 4 weeks. Risk and control windows were selected based on the expected temporal relationship between MTX use and peak onset of these symptoms. Assessments included PROMIS short forms for same-day Fatigue, same-day Nausea/Vomiting, and Patient Global. Descriptive statistics were conducted using paired t-tests two-way comparisons. Within-person change in PROMIS scores between the risk (1-2 days after MTX) and control (4-6 days after MTX) windows were analyzed using mixed models for repeated measures, stratified on whether pts reported fatigue or nausea with MTX at baseline. Recruitment for this study is ongoing.
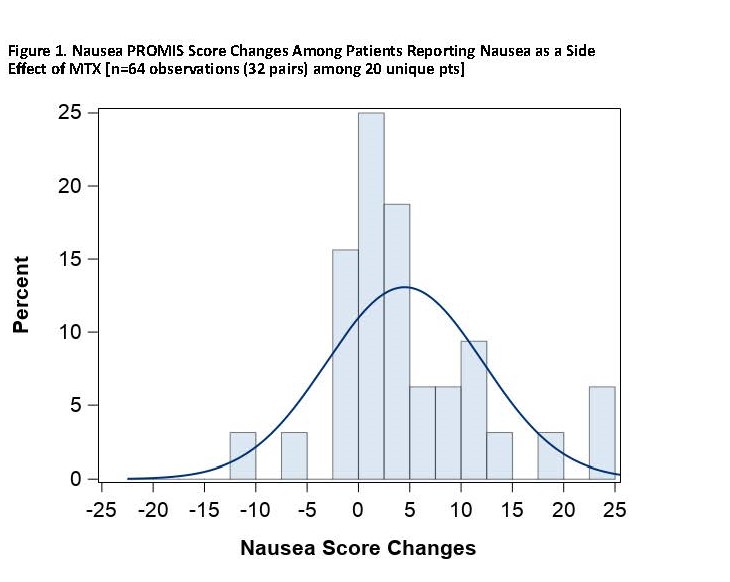
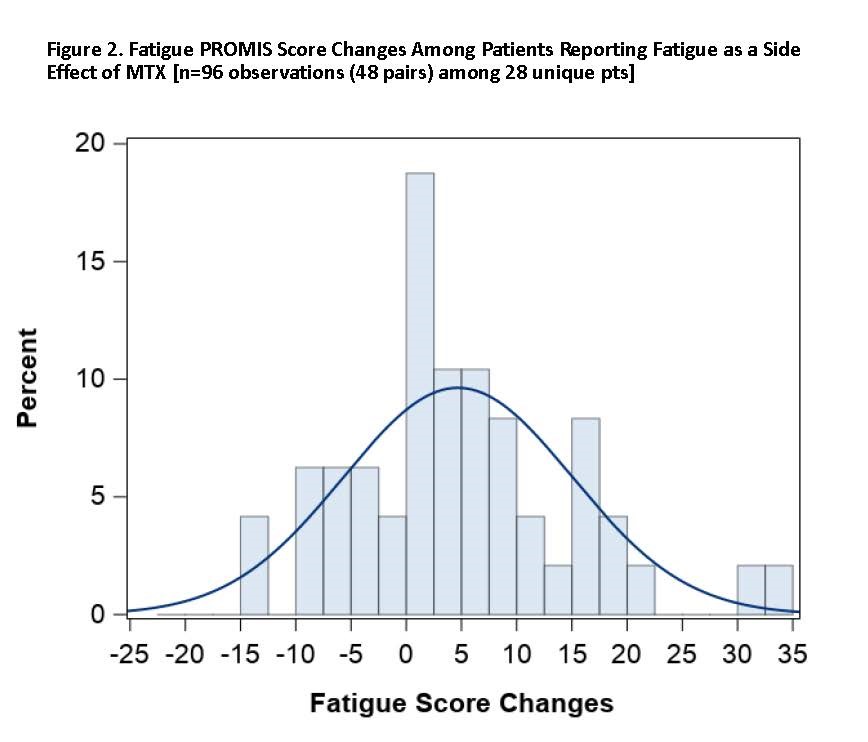
Results: As of December 2019, 91 pts had participated, of whom 76.9% were living with RA and 28.6% with PsA, with mean baseline PROMIS Patient Global score (SD) of 39.5 (7.1). Mean age (SD) was 50.9 (12.0) years, 84.6% female, 92.3% White, with mean BMI 33.7 (8.8). Mean duration of MTX treatment among current users was 2.1 (2.8) years. Among pts, 41.8% were on a biologic DMARD and 58.2% on non-biologic DMARDs only. Among pts reporting baseline nausea (n=30, 33.0%) where paired within-week measures were observed (n=64 observations among 20 pts), the mean increase in the PROMIS Nausea score was 4.5 units (adjusted p=0.003). Among those reporting MTX-associated fatigue (n=39, 42.9%) as a side effect of MTX on their baseline survey where paired within-week measures were observed (n=96 observations among 28 pts), the mean increase in PROMIS Fatigue was 4.7 (adjusted p=0.004) units. In those pts, the proportion of pts with worsened nausea and fatigue with minimally important difference of >5 units was 40.0% (nausea), and 60.7% (fatigue) [Figures 1 and 2].
Conclusion: People taking MTX to manage RA or PsA commonly experience bothersome side effects, notably fatigue and nausea, that are temporally related to weekly MTX dosing. In this sample, one-third or more of pts were bothered by nausea or fatigue shortly after MTX dosing, many of them with clinically meaningful symptoms.
To cite this abstract in AMA style:
Nowell W, Karis E, Gavigan K, Stradford L, Stryker S, Yun H, Venkatachalam S, Kricorian G, Chen L, Zhao H, Xie F, Curtis J. Changes in Patient-Reported Outcome (PRO) Scores for Nausea and Fatigue Following Weekly Methotrexate Dose in a Real-World Sample of RA and PsA Patients in the ArthritisPower Registry [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/changes-in-patient-reported-outcome-pro-scores-for-nausea-and-fatigue-following-weekly-methotrexate-dose-in-a-real-world-sample-of-ra-and-psa-patients-in-the-arthritispower-registry/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/changes-in-patient-reported-outcome-pro-scores-for-nausea-and-fatigue-following-weekly-methotrexate-dose-in-a-real-world-sample-of-ra-and-psa-patients-in-the-arthritispower-registry/