Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: In the SENSCIS trial in patients with SSc-ILD, nintedanib reduced the rate of decline in forced vital capacity (FVC) (mL/year) over 52 weeks by 44% compared with placebo. The effects of nintedanib on markers of lung damage on high-resolution computed tomography (HRCT) were assessed in a sub-study.
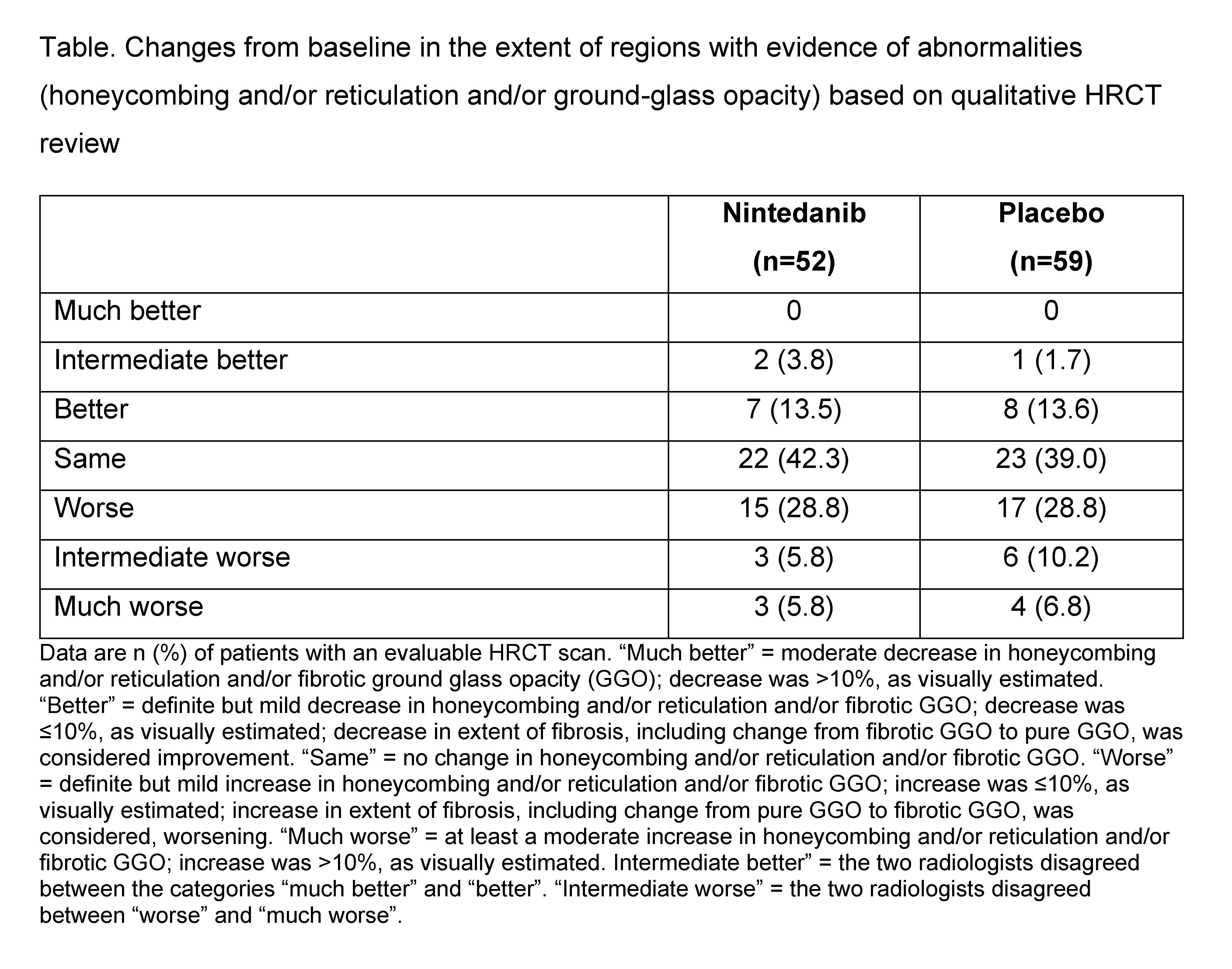
Methods: Patients enrolled in the SENSCIS trial had SSc with onset of first non-Raynaud symptom ≤7 years before screening and an extent of fibrotic ILD ≥10% on HRCT (based on assessment of the whole lung). Patients were randomized to receive nintedanib or placebo. In a sub-study, HRCT images were assessed at baseline and at week 52 or 60. The extent of regions with evidence of abnormalities (honeycombing and/or reticulation and/or ground-glass opacity) was assessed in both lungs and changes from baseline were categorized by two radiologists as “much better”, “better”, “same”, “worse”, “much worse” or “unknown”. Disagreement between the radiologists in the categories “much better” or “better” and “worse” or “much worse” were considered “intermediate better” or “intermediate worse”. An ordinal logistic regression analysis (proportional odds model) adjusted for anti-topoisomerase antibody I status was used to compare changes between the treatment groups. Changes from baseline in a quantitative fibrosis score, a measure of the extent (%) of reticular patterns with architectural distortion, were assessed using data-driven texture analysis. Analyses were conducted in patients who received trial medication up to at least week 24 and had an evaluable HRCT scan at week 52/60.
Results: Of 576 patients who participated in the SENSCIS trial, 150 participated in the HRCT sub-study (nintedanib 73, placebo 77). The rate of decline in FVC over 52 weeks was similar in the overall trial population and in patients who participated in the HRCT sub-study (Figure). Compared with the placebo group (n=59), a lower proportion of patients in the nintedanib group (n=52) had a worsening in qualitative parameters (i.e., “worse”, “intermediate worse”, or “much worse”) from baseline to week 52–60 (40.4% vs 45.8%) (Table). Ordinal logistic regression analysis demonstrated a numerically greater risk of worsening in qualitative parameters in the placebo group compared with the nintedanib group (OR 1.24 [95% CI: 0.63, 2.47]; p=0.53). Adjusted mean (SE) changes from baseline in quantitative fibrosis score were 2.50 (0.88) % in the nintedanib group (n=23) and 2.80 (0.73) % in the placebo group (n=31) (difference: −0.31% [95% CI: −2.36, 1.75]; p=0.77).
Conclusion: In a sub-study of the SENSCIS trial, qualitative and quantitative changes on HRCT over 52–60 weeks were small. Numerical but non-significant trends towards less worsening were observed in patients treated with nintedanib versus placebo. These analyses were limited by the small number of patients who had an evaluable HRCT scan at the follow-up visit.
To cite this abstract in AMA style:
Humphries S, Hachulla E, Hamblin M, Ogura T, Wormanns D, Ittrich C, Risse F, Alves M, Gahlemann M, Lynch D. Changes in Imaging Markers in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease (SSc-ILD) Treated with Nintedanib: Sub-Study of the SENSCIS Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/changes-in-imaging-markers-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-ssc-ild-treated-with-nintedanib-sub-study-of-the-senscis-trial/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/changes-in-imaging-markers-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-ssc-ild-treated-with-nintedanib-sub-study-of-the-senscis-trial/