Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Current recommendations suggest treatment escalation for juvenile idiopathic arthritis (JIA) until the disease activity target is reached, ideally inactive or low disease activity. Our objective was to compare patient reported outcomes (PRO) 6 months after maximally tolerated disease activity level.
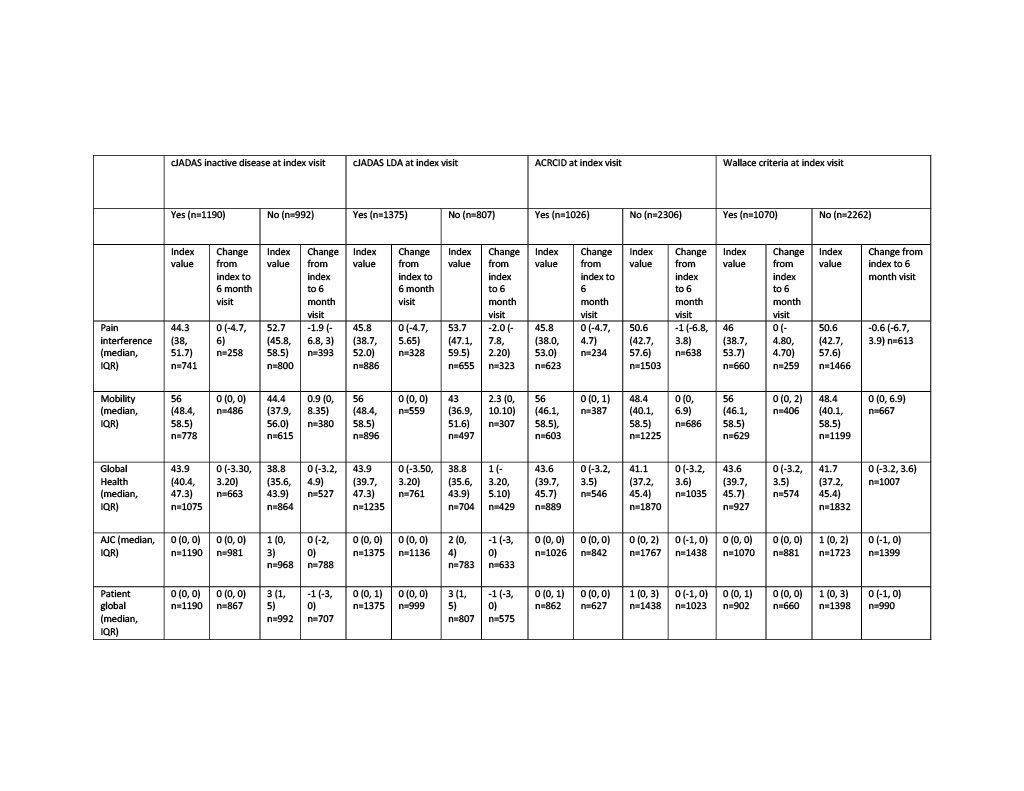
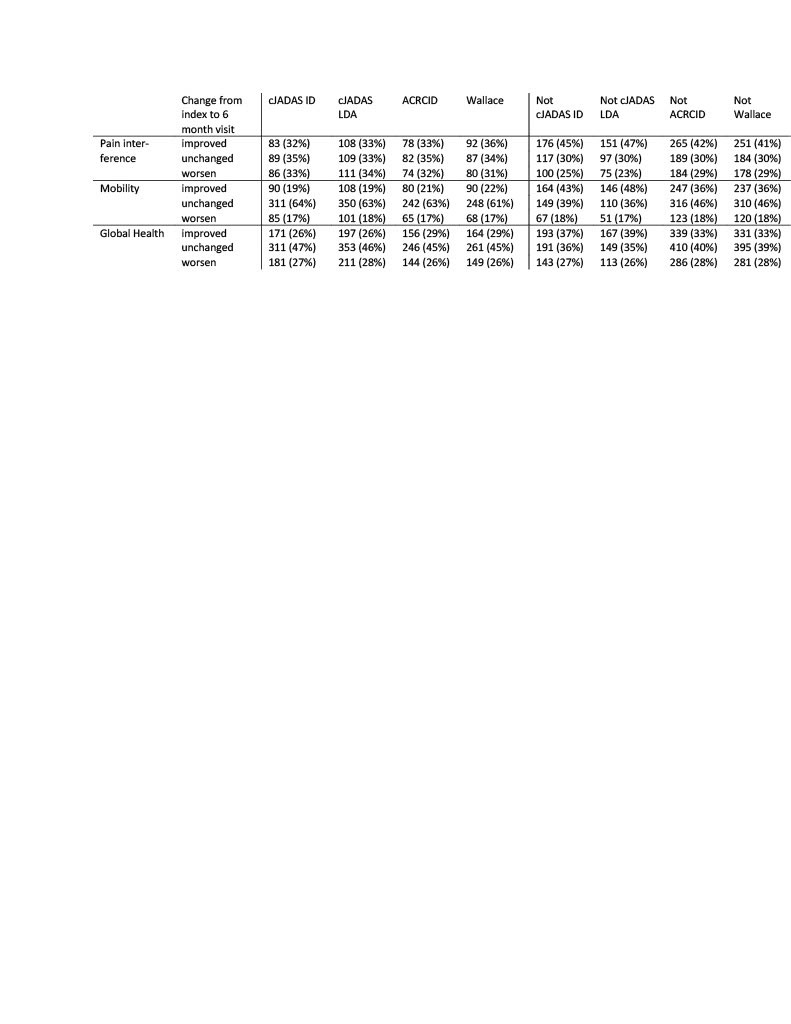
Methods: We included all individuals enrolled with non-systemic JIA in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry from 2015-2019. The maximally tolerated disease activity level was determined at registry visits where there had been no medication change for >180 days prior to and 60 days following the visit (index visit). Individuals could contribute more than one observation but were excluded for a medication change between the index and 6 month follow up visit. Individuals were considered to have polyarticular (poly) course if cumulative joint count was >4 and oligoarticular (oligo) course if total joint count was <4 at the index visit. Disease activity was classified based on the clinical Juvenile Arthritis Disease Activity Score (cJADAS) inactive disease (ID; oligo <1.1, poly <2.5), cJADAS low disease activity (LDA; oligo <2, poly <3.8), the American College of Rheumatology (ACR) provisional criteria for clinical inactive disease (ACRCID) and the ACR preliminary criteria for clinical inactive disease (Wallace). Change in outcome variables (Patient Reported Outcomes Measurement Information System [PROMIS] pain interference, mobility, and pediatric global health, active joint count [AJC], patient global assessment score, and childhood health assessment questionnaire [CHAQ]) from index to 6 month visit was stratified by disease activity status at the index visit and presented as absolute change (Table 1). Relative improvement or worsening in outcome variables was determined by measure specific minimal clinical important difference (MCID) and the association with tolerated disease activity state was compared by descriptive statistics.
Results: There were 6,235 individuals with JIA and 16,240 observations included. The tolerated disease activity state was cJADAS ID in 35%, cJADAS LDA in 41%, ACRCID in 29%, and Wallace criteria in 33% of observations. At the index visit for all patients the median (interquartile range; IQR) pain interference was 49 (40.6, 56.6, n=6359), mobility was 56 (43, 58.5, n=6670), and global health was 42.1 (37.9, 45.7, n=8445). cJADAS ID, cJADAS LDA, ACRCID, and Wallace were all associated with better pain interference, mobility and global health scores at the index visit compared to not meeting criteria for ID or LDA, and the median change at 6 months was less than the MCID for all groups. The relative worsening or improvement in each PROMIS measure was similar between ID and LDA definitions.
Conclusion: Individuals with JIA often have higher than ID or LDA at the time of no medication change and have a lower global health than population norms. There were similar frequency of worsening for all measures and tolerated disease activity states; these results do not suggest preference of one criteria set over another. Individuals who do not meet ID or LDA criteria are more likely to improve than worsen after 6 months.
To cite this abstract in AMA style:
Mannion M, Xie F, Beukelman T, Curtis J, for the CARRA Registry Investigators. Change in Short Term Outcomes Following Tolerated Disease Activity Level for Individuals with Juvenile Idiopathic Arthritis in the Childhood Arthritis and Rheumatology Research Alliance Registry [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/change-in-short-term-outcomes-following-tolerated-disease-activity-level-for-individuals-with-juvenile-idiopathic-arthritis-in-the-childhood-arthritis-and-rheumatology-research-alliance-registry/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/change-in-short-term-outcomes-following-tolerated-disease-activity-level-for-individuals-with-juvenile-idiopathic-arthritis-in-the-childhood-arthritis-and-rheumatology-research-alliance-registry/