Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Remote Therapeutic Monitoring (RTM) connects patients to their healthcare provider using a smartphone app with or without a physiologic biosensor device in an insurance-reimbursable program that compensates physicians each month for their patients’ use of this technology and its associated monitoring by office staff. RTM has gained significant attention during the COVID-19 pandemic for managing chronic conditions. This study evaluated RTM’s feasibility for musculoskeletal diseases (rheumatoid arthritis, psoriatic arthritis, and axial spondyloarthritis) post-pandemic.
Methods: RTM used the FDA-cleared medical device PatientSpot registry, from 4/3/23-1/1/24. Customized RTM pathways were implemented for patients across 25 US rheumatology clinics (Image 1). Enrollment around routine visits where office staff provided a link to a registration page. Patients used the PatientSpot mobile app (previously ArthritisPower) to fill out Patient Reported Outcome Measures (PROM) longitudinally, monitored as part of standard care.
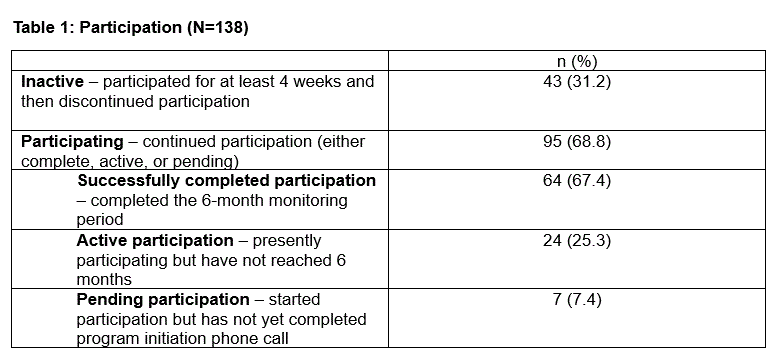
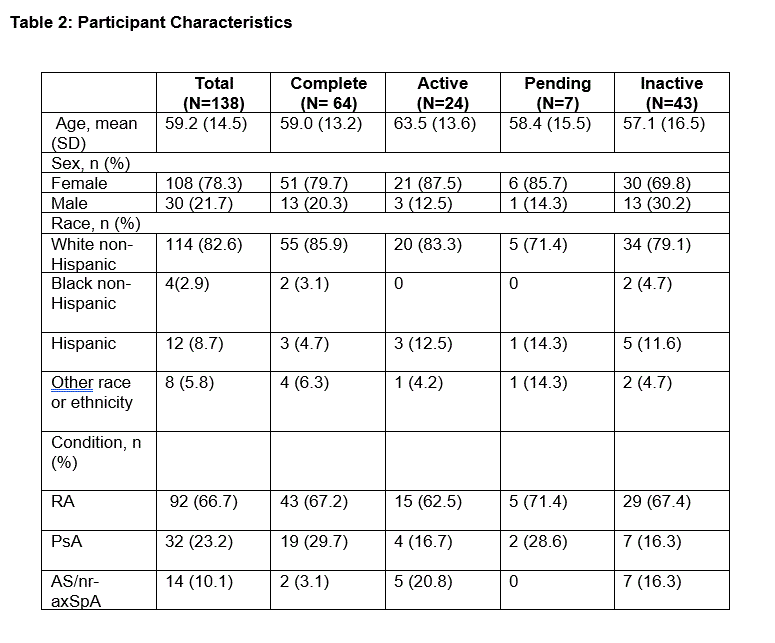
Results: Of 7,960 registration page visits, 832 individuals agreed to participate (10.5%). Of these, 504 registered for RTM (60.6%), with 138 completing the process (27.4%). Among 138 participants, 31.2% became inactive after at least four weeks while the remaining 95 participants (68.8%) continued in the program. Of those 95 participants, 64 (67.4%) completed their participation, 24 (25.3%) were actively participating and not yet at six months, and 7 (7.4%) were pending (Table 1). Mean participant age was 59.2 years and 78.3% were female. The majority (82.6%) were White non-Hispanic, 8.7% Hispanic, 2.9% Black non-Hispanic, and 5.8% other race/ethnicity. Conditions included rheumatoid arthritis (66.7%), psoriatic arthritis (23.2%), and ankylosing spondylitis/non-radiographic axial spondyloarthritis (10.1%) (see Table 2 for characteristics). Over 24 weeks, patient-reported outcomes included pain interference, fatigue, physical function, social activity satisfaction, sleep disturbance, disease activity, and quality of life.
All participants were asked to fill out a satisfaction survey after 4 weeks of participating in the program. Participant feedback (n=66) showed high satisfaction with the RTM program: 36.4% very satisfied and 53.0% somewhat satisfied. Improved follow-up from doctors (42.4%) and monitoring disease changes over time (24.2%) were noted as major benefits. For those with low uptake, 42.4% frequently forgot to answer questions, and 27.3% struggled to find time. Preferred reminders were emails (47.0%) and app notifications (43.9%).
Conclusion: The potential for RTM to serve as an effective tool for monitoring patients with rheumatic and musculoskeletal conditions between office visits by tracking PROMs remains promising. As the healthcare landscape continues to evolve post-pandemic, making RTM both accessible and manageable for patients and providers is essential for collaborative management of disease. Addressing barriers related to patient compliance and staff workload is necessary for optimal uptake. Ongoing work is refining RTM pathways to optimize for specific clinical scenarios and add new diseases (e.g. osteoporosis).
To cite this abstract in AMA style:
Degrassi A, Curtis J, Curtis D, Gavigan K, Stradford L, Fritz S, Sodhi S, Venkatachalam S. Challenges and Opportunities in Post-Pandemic Remote Therapeutic Monitoring for Musculoskeletal Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/challenges-and-opportunities-in-post-pandemic-remote-therapeutic-monitoring-for-musculoskeletal-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/challenges-and-opportunities-in-post-pandemic-remote-therapeutic-monitoring-for-musculoskeletal-disease/