Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The optimal first-line treatment of patients with early rheumatoid arthritis (eRA) is not established.
Methods: In this investigator-initiated, randomized, open-label study (NCT01491815), patients with treatment-naïve eRA with DAS28 >3.2 and RF+/ACPA+/CRP >10mg/L, were randomized 1:1:1:1 to methotrexate (MTX) combined with: 1) oral prednisolone (tapered quickly; discontinued at w36); or: sulphasalazine, hydroxychloroquine and mandatory intra-articular (IA) glucocorticoid injections in swollen joints (ACT); 2) certolizumab-pegol (CZP); 3) abatacept (ABA) or 4) tocilizumab (TCZ). IA glucocorticoid was allowed in all arms except during w20-24 and w44-48. The primary outcome Clinical Disease Activity Index (CDAI) remission and key secondary outcomes at 24 weeks have previously been published (Hetland ML et al, BMJ 2020;371:m4328). Differences between ACT and each of the 3 biological therapies for PRO (pain, patient´s global assessment, HAQ-DI, fatigue (VAS and FACIT) at 48 weeks were tested and descriptive data presented with 95% CI. Longitudinal data points were analyzed using a linear mixed model analysis. The continuous and dichotomous PRO data were adjusted for country, sex and anti-citrullinated protein antibody status. For continuous data, additional adjustment included baseline values of PRO endpoint and treatment arm and time as well as their interaction as categorical covariates. MCID for pain and fatigue were defined as: ≥ 10-point decrease for VAS pain (Strand V et al; J Rheumatol 2011;38;1720-7) and ≥10-point decrease for VAS Fatigue (Wells et al, J Rheumatol 2007:34(2);280-9), respectively.
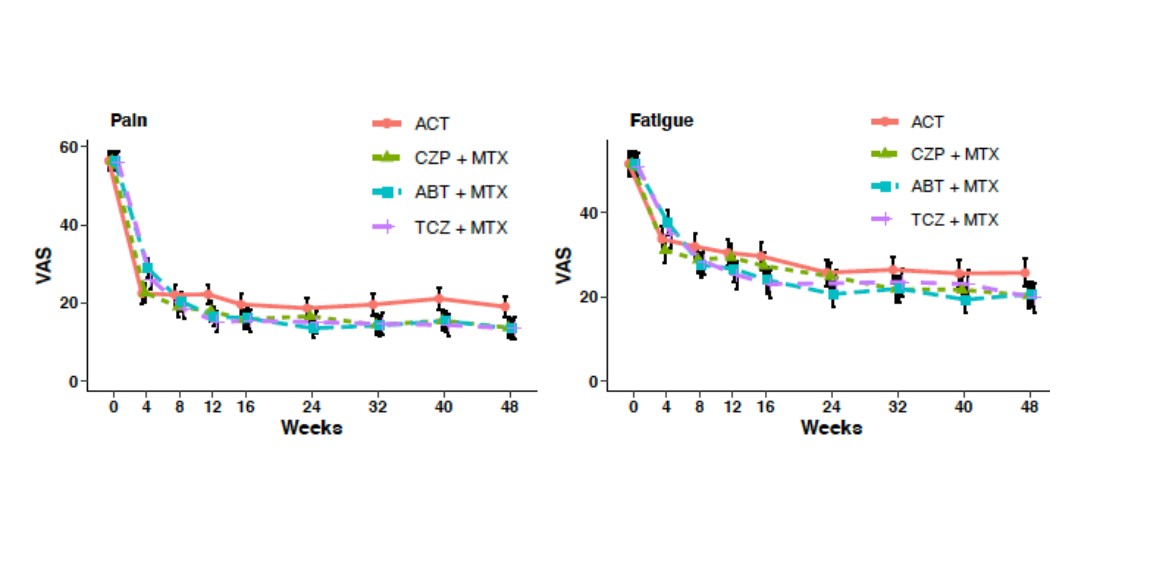
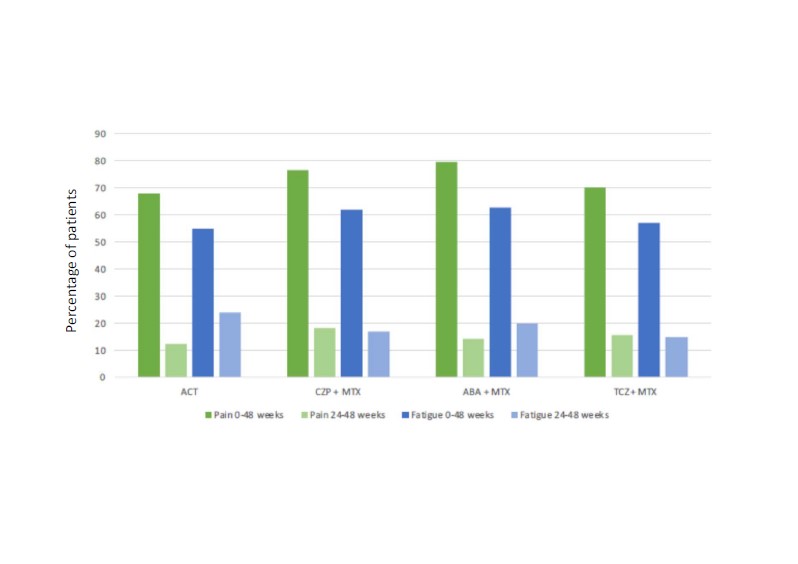
Results: 812 patients were randomized. Baseline characteristics and adjusted baseline and 48-week PRO data are shown in table 1. In the longitudinal analysis there was a trend for greater improvement of pain and fatigue in biological groups compared to ACT (figure 1). Adjusted for baseline values, pain levels and physician global assessment were numerically lower in all biological groups compared to ACT at week 48. The percentage of patients reporting improvements above MCID for pain and fatigue (from baseline until 48 weeks) were numerically higher in biological arms compared to ACT (% and 95% CI), as follows: MCID for pain: ACT 68% (61, 74); CZP+MTX 77% (71, 82); ABA+MTX 80% (74, 85); TCZ+MTX 70% (64, 77); and for fatigue: ACT 55% (48, 62); CZP+MTX 62% (55, 69); ABA+MTX 63% (56, 69); TCZ+MTX 57% (59, 64) (figure 2).
Conclusion: All four different modes of action lead to marked improvements of PROs after 48 weeks. Compared with csDMARD+glucocorticoid treatment pain levels were lower in all biological arms at week 48. Likewise, the percentages of patients with clinically important improvements for pain and fatigue were numerically higher in the treatment arms with biological vs. conventional treatment.
To cite this abstract in AMA style:
Lampa J, Nordstrom D, van Vollenhoven R, Hetland M, Haavardsholm E, Østergaard M, Rudin A, Schrumpf Heiberg M, Nurmohamed M, Gudbjornsson B, Lend K, Hørslev-Petersen K, Sokka-Isler T, Grondal G, Krabbe S, Lindqvist J, Hultgård Ekwall A, Glinatsi D, Kapetanovic M, Gentline C, Aga A, Relas H, Lorenzen T, Cagnotto G, Back J, Hendricks O, Dijkshoorn B, Öberg K, Ljoså M, Brodin E, Lindegaard H, Söderbergh A, Rizk M, Kastbom A, Larsson P, Uhrenholt L, Just S, Stevens D, Laurberg T, Bakland G, Olsen I, Sexton J, Uhlig T. Certolizumab-pegol, Abatacept, Tocilizumab or Active Conventional Therapy in Early Rheumatoid Arthritis: 48 Week Patient-reported Outcomes of the NORD-STAR Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/certolizumab-pegol-abatacept-tocilizumab-or-active-conventional-therapy-in-early-rheumatoid-arthritis-48-week-patient-reported-outcomes-of-the-nord-star-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/certolizumab-pegol-abatacept-tocilizumab-or-active-conventional-therapy-in-early-rheumatoid-arthritis-48-week-patient-reported-outcomes-of-the-nord-star-trial/