Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Fasinumab, a newly developed anti-nerve growth factor monoclonal antibody, is currently under investigation for its potential to alleviate symptoms in osteoarthritis patients. This meta-analysis aims to analyze the efficacy and safety of Fasinumab compared to placebo in osteoarthritis patients.
Methods: PubMed (MEDLINE), Cochrane CENTRAL, ClinicalTrials.gov, and Google Scholar were searched for double-blinded Randomized Control Trials (RCTs) comparing Fasinumab with placebo till February 2024. Primary outcomes were WOMAC pain, WOMAC function, and Patient Global Assessment (PGA) scores. Cochrane Risk of Bias 2 was used for quality assessment. Statistical analysis was carried out using a Random Effects model. Subgroups based on dosages were also made.
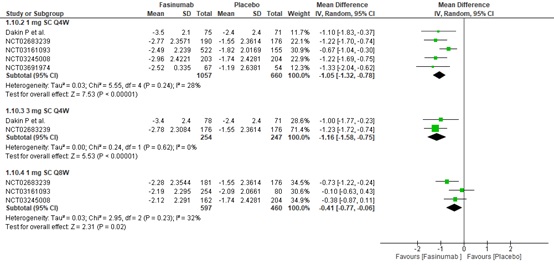
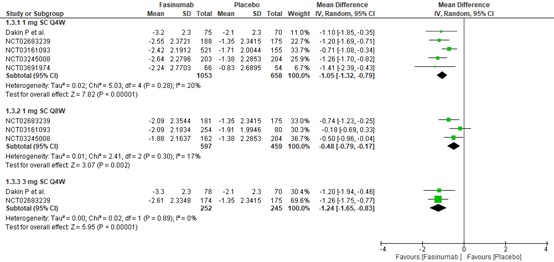
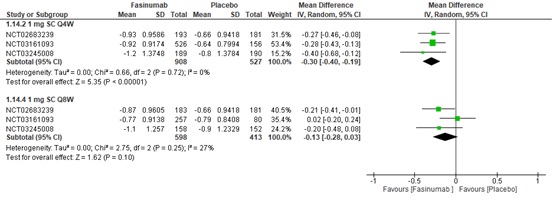
Results: Six RCTs involving 9,429 participants were included. At 16 weeks, 1 mg Subcutaneous (SC) Every four weeks (Q4W), 1 mg SC Every eight weeks (Q8W), and 3 mg SC Q4W showed a greater statistically significant reduction in WOMAC pain and function scores compared to placebo. However, according to minimal clinically important differences (MCIDs), only 1mg SC Q4W and 3mg SC Q4W may provide clinically meaningful reductions in WOMAC pain ([MD: -1.05, (95% C.I. -1.32 to -0.78), p< 0.00001, I²=28%] and [MD: -1.16, (95% C.I. -1.58 to -0.75), p< 0.00001, I²=0%], respectively) and function scores ([-1.05 (95% C.I. -1.32 to -0.79), p< 0.00001, I²=20%] and [MD: -1.24, (95% C.I. -1.65 to -0.83), p< 0.00001, I²=0%], respectively) more than placebo. A 1 mg SC Q4W dosage regimen is also favored over a placebo in improving PGA scores at 16 weeks. However, Fasinumab use increases the risk of adverse events, including Joint-replacement surgery and Adjudicated Arthropathy.
Conclusion: Specific Fasinumab dosages showed greater efficacy in alleviating pain and improving function and PGA scores compared to placebo. However, Fasinumab use may be associated with increased health detriments, such as increased risk of adjudicated arthropathy and all-cause joint replacement.
To cite this abstract in AMA style:
Khan M, Moeen W, Shabbar Banatwala U, Usman M, Mahmood U, Ahmed Fatmi S, Muneeb Akhtar S, Shahzad H, Ibrahim M, Ahmed N, Sahito B, Asghar M. Certain Dosages of Fasinumab Provides Superior Benefits over Placebo in Mitigating Osteoarthritis Symptoms Albeit at Increased Health Detriments: A Systematic Review and Meta-Analysis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/certain-dosages-of-fasinumab-provides-superior-benefits-over-placebo-in-mitigating-osteoarthritis-symptoms-albeit-at-increased-health-detriments-a-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/certain-dosages-of-fasinumab-provides-superior-benefits-over-placebo-in-mitigating-osteoarthritis-symptoms-albeit-at-increased-health-detriments-a-systematic-review-and-meta-analysis/