Session Information
Date: Monday, November 14, 2022
Title: Abstracts: T Cell Biology and Targets in Autoimmune and Inflammatory Disease
Session Type: Abstract Session
Session Time: 3:00PM-4:00PM
Background/Purpose: Patients with cancer exhibit increased mortality from SARS-CoV-2 infection compared to the general population. SARS-CoV-2 mRNA vaccination in healthy individuals generates effective and long-lasting immune protection against COVID-19. PD-1 is highly expressed on the surface of T follicular helper cells that play a central role in T cell dependent B cell vaccine responses. Here, we sought to investigate the cellular and humoral responses in patients on PD-1 immunotherapy after SARS-CoV-2 mRNA vaccination, with a particular focus on patients developing immune-related adverse events (irAEs).
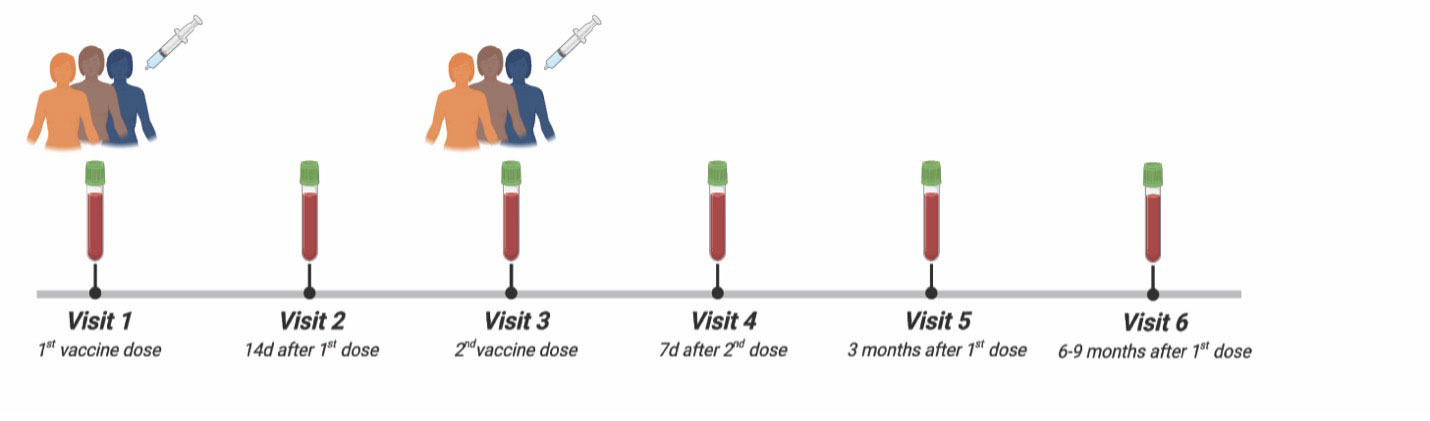
Methods: We investigated the longitudinal induction of antigen-specific antibody, B cell and T cell responses in patients with melanoma on anti-PD-1 immunotherapy (aPD-1, n = 44) receiving a 2-dose SARS-CoV-2 mRNA vaccine regimen during 6 distinct timepoints over the course of 9 months (Figure 1). We compared these responses against an established cohort of healthy control individuals (HC, n=45). All individuals included in this study were not previously infected with SARS-CoV-2. Incidence of irAEs in the aPD-1 patients was determined with retrospective chart review.
Results: After a 2-dose mRNA vaccine regimen, a-PD1 patients had lower levels of anti-Spike IgG after the first vaccine dose (V2) and lower levels of anti-Receptor Binding Domain (RBD) IgG after the first and second dose (V2 and V4) compared to healthy controls (Figure 2). Within the aPD-1 patient group, patients with irAEs demonstrated a trend towards lower anti-Spike and anti-RBD IgG compared to patients with no irAEs (Figure 3). Vaccine-specific memory B cells (mBCs) recognizing Spike and RBD were generated in patients on aPD-1 therapies to an extent equivalent to healthy controls. The mBCs from aPD-1 patients efficiently recognized the Alpha, Beta and Delta variants of concern (VOC) of SARS-CoV-2 but exhibited diminished recognition of the Omicron variant at 2 months after the 2nd vaccination (V5). This defect improved over time, with mBCs from aPD-1 patients recognizing the Omicron VOC at levels similar to the control group by 6-9 months following primary vaccination (V6). Finally, using a peptide pool of the wild-type Spike protein, we found that all aPD-1 patients generated antigen-specific CD4 and CD8 T-cell responses following vaccination. Nevertheless, when tested with an omicron-specific peptide pool, the abundance of antigen-specific CD4 T cells, but not CD8 T cells, from patients on aPD-1 immunotherapy was lower than that of healthy controls.
Conclusion: These results indicated that patients on aPD-1 immunotherapeutics generate productive humoral and cellular vaccine-specific responses against the wild-type Spike protein following SARS-CoV-2 mRNA vaccination. However, they also demonstrated diminished B cell and CD4 T cell recognition of the immune-evasive Omicron variant. In addition, patients with irAEs showed some of the lower serological responses among all the individuals tested. The qualitative defects seen in patients on aPD-1 therapy highlight the importance of the PD-1 pathway in promoting fully functional vaccine responses, suggesting possibly a more pressing need for these patients to receive booster vaccinations.
To cite this abstract in AMA style:
Apostolidis S, Coutifaris P, Painter M, Pattekar A, Goel R, mathew d, Meng W, Wang K, Fulmer B, Corrigan A, Lundgreen K, Drapeau E, Oldridge D, Giles J, Baxter A, Manne S, Mckeague M, Flowers A, Kuthuru O, Long S, Dougherty J, Gouma S, Williams J, McLaughlin M, Adamski S, Grifoni A, Weiskopf D, Sette A, Hensley S, Bates P, Luning Prak E, Vella L, Karakousis G, Amaravadi R, McGettigan S, Kreider K, Mitchell T, Greenplate A, Schuchter L, Huang A, Wherry E. Cellular and Humoral Responses Following SARS-CoV-2 mRNA Vaccination in Patients on PD-1 Immunotherapy with irAEs [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/cellular-and-humoral-responses-following-sars-cov-2-mrna-vaccination-in-patients-on-pd-1-immunotherapy-with-iraes/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cellular-and-humoral-responses-following-sars-cov-2-mrna-vaccination-in-patients-on-pd-1-immunotherapy-with-iraes/