Session Information
Date: Sunday, November 7, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster II: Manifestations (0855–0896)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: SLE is a multisystem autoimmune disease that displays diverse manifestations that can be categorized into two broad types. Type 1 SLE activity includes inflammatory manifestations such as arthritis and nephritis. Type 2 SLE activity, which has a less clear relationship to inflammation, includes findings such as fatigue and widespread pain and can be assessed using patient-reported outcome measures (PROs). Cell bound-activation products (CB-CAPs), erythrocyte-bound C4d (EC4d), and B-lymphocyte-bound C4d (BC4d) in a multi-analyte assay with algorithm (MAP) provide valuable diagnostic information in SLE. In this study, we evaluated EC4d, BC4d, and MAP as markers of current Type 1 SLE activity.
Methods: This was a cross-sectional study of SLE patients (ACR 1997 or SLICC criteria) from June 2020 to March 2021. ANA and other autoantibodies were measured by ELISA. Anti-dsDNA was determined by immunofluorescence using Crithidia luciliae. CB-CAPs were measured by flow cytometry. The multi-analyte assay panel with algorithm (MAP) was determined using a 2-tier algorithm.
We used SLEDAI, the Polysymptomatic Distress Score (PSD, patient-reported pain and symptoms), and a PGA (physician’s global assessment; a 3-point visual analog scale) for Type 1 and for Type 2 SLE activity to define 3 bookended patient groups based on current SLE activity:
- Type 1 SLE activity: SLE Disease Activity Index (SLEDAI-2k) ≥6, clinical SLEDAI ≥4, renal SLEDAI ≥4 or Type 1 PGA ≥1.5, with or without Type 2 SLE activity
- Type 2 SLE: PSD score ≥8, Type 2 PGA ≥1.5, SLEDAI=0, and Type 1 PGA ≤0.5
- Minimal SLE: PSD score of ≤3, SLEDAI=0, Type 1 PGA ≤0.5 and Type 2 PGA ≤0.5.
Differences across groups were analyzed by Fisher’s exact test. Logistic regression analysis examined predictors of Type 1 activity.
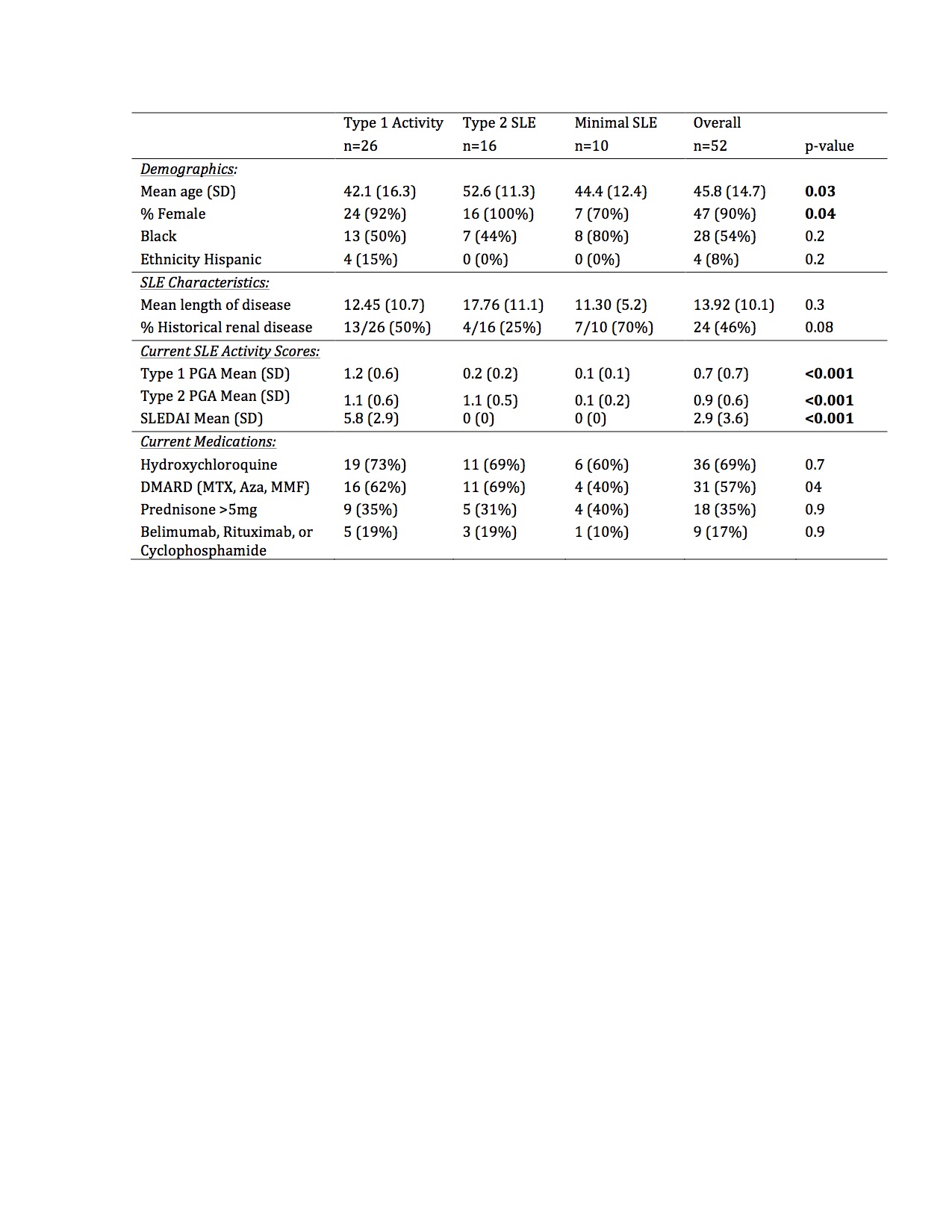
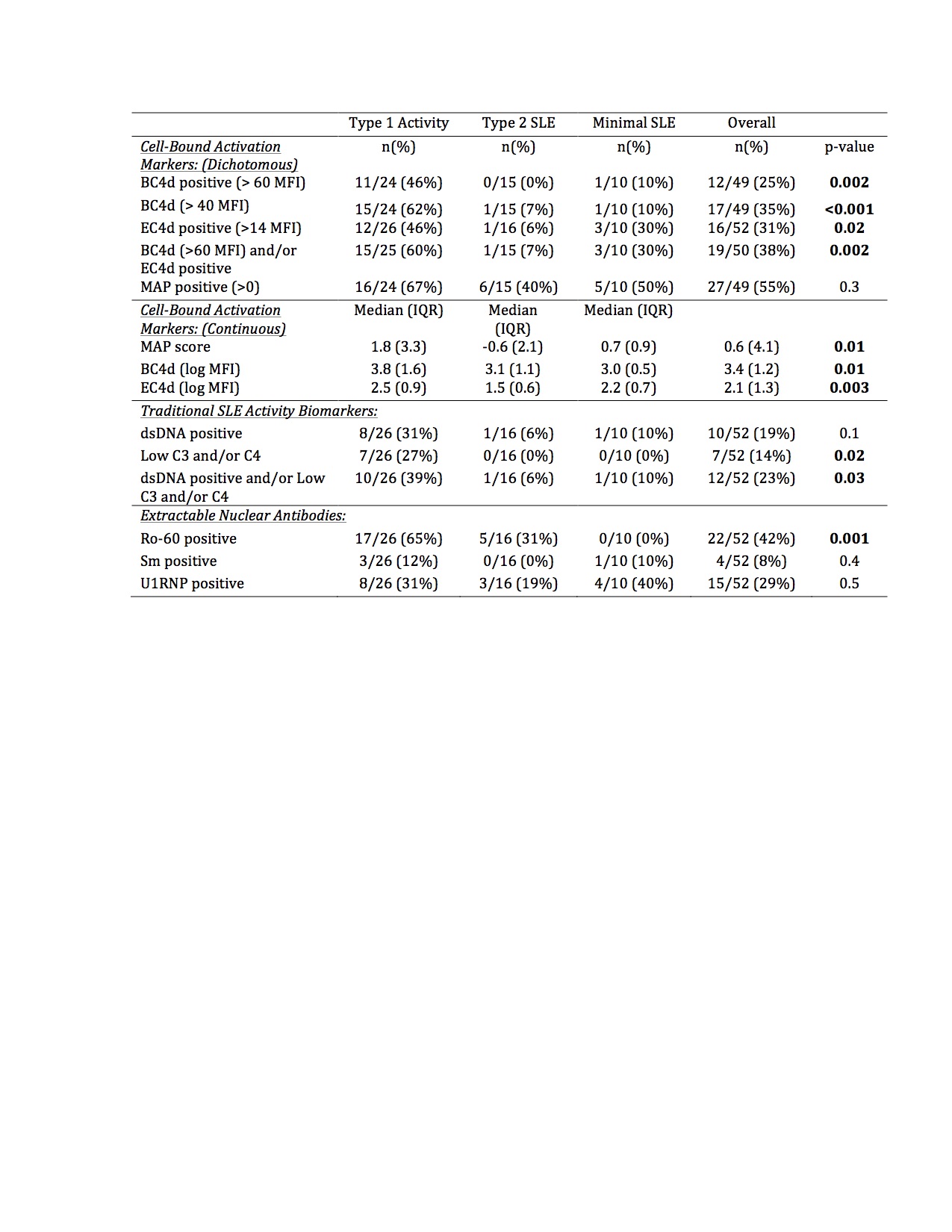
Results: In this bookended cohort of 52 patients (90% female, 54% Black, mean age 46 years), 50% had current Type 1 activity, 31% had Type 2 SLE without Type 1 activity, and 19% had minimal SLE activity. Patients with Type 1 activity were younger, but there was no difference in race, ethnicity, length of disease, historical renal disease, hydroxychloroquine, or immunosuppression use across the groups (Table 1). The median MAP score and BC4d and EC4d levels were significantly higher in patients with current Type 1 activity (Table 2). Additionally, BC4d, EC4d, and Ro-60 were more frequently positive in patients with current Type 1 activity. Only 39% of patients with Type 1 SLE had positive dsDNA and/or low complement. MAP positivity, Sm, and U1-RNP did not distinguish between the groups. In a predictive model, the presence of BC4d >40 or Ro-60 >20 resulted in an 84.6% sensitivity, 73.1% specificity, and diagnostic odds ratio of 14.9 for Type 1 SLE activity.
Conclusion: CB-CAPs may serve as effective biomarkers to categorize current SLE activity using the Type 1 and Type 2 model. MAP scores as well as BC4d, EC4d, and Ro-60 positivity, but not traditional SLE-related antibodies, differentiated current Type 1 SLE activity from Type 2 SLE and low disease activity patients. The addition of CB-CAPs to PROs assessing Type 2 SLE enhances the rheumatologist’s ability to categorize more completely the spectrum of SLE symptomatology.
To cite this abstract in AMA style:
Rogers J, Bloch R, Eudy A, Pisetsky D, Alexander R, Conklin J, Sun K, Sadun R, Criscione-Schreiber L, Doss J, Clowse M. Cell-bound Complement Activation Products (CB-CAPs) Predicts Type 1 SLE Activity [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/cell-bound-complement-activation-products-cb-caps-predicts-type-1-sle-activity/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cell-bound-complement-activation-products-cb-caps-predicts-type-1-sle-activity/