Session Information
Date: Sunday, November 10, 2019
Title: SLE – Animal Models Poster
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: T cells are an important contributor the pathogenesis of SLE and its various end organ manifestations. Thus, they present themselves as potential therapeutic targets; however, the optimal approach to targeting T cells in SLE still remains under investigation. CD6 is a co-stimulatory receptor, predominantly expressed on T cells, that binds to activated leukocyte cell adhesion molecule (ALCAM), a ligand expressed on antigen presentation cells and various epithelial and endothelial tissues. The CD6-ALCAM pathway plays an integral role in modulating T cell activation, proliferation, differentiation and trafficking. In this study, we assessed the expression of CD6 and ALCAM within the context of a murine model of SLE, and then subsequently targeted this signaling axis to determine its role in the pathogenesis of disease.
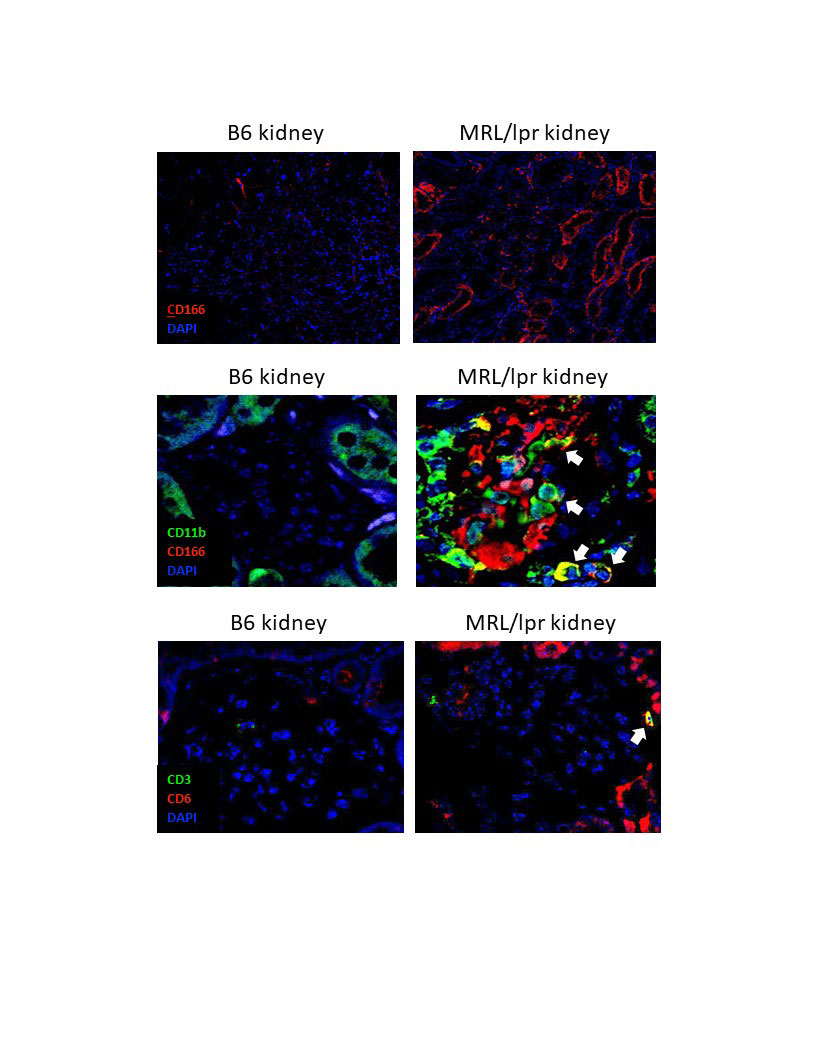
Methods: The MRL/lpr mouse is a spontaneous murine model of SLE, which exhibits systemic autoimmunity, nephritis, and skin disease. In an initial experiment, 6 month old MRL/lpr mice and B6 kidneys were stained for the presence of both ALCAM and CD6. In a separate experiment, female MRL/lpr mice were aged to 9-10 weeks of age, and then treated with either anti-CD6 monoclonal antibody (60 ug/dose, IP twice per week), isotype control (60 ug/dose, twice per week), or cyclophosphamide (25 mg/kg, once per week). We also included a no treatment group, and a group of MRL/MpJ mice, a congenic healthy control strain. Baseline levels of anti-DNA antibodies, weight, and proteinuria in the MRL/lpr groups were similar. Mice were monitored weekly for proteinuria, lymph node swelling, and macroscopic skin lesions.
Results: MRL/lpr mice at 6 months of age show increased levels of ALCAM expression in their kidneys, both within their tubules and glomeruli, compared to B6 healthy control mice (shown are images representative of 3 mice per group). Additionally, macrophages infiltrating into the glomeruli of MRL/lpr mice were ALCAM+ (white arrows, top panel) and were paired with a concomitant increase in CD6+ T cell infiltration (white arrows, bottom panel). Consequently, in a separate study, we blocked this signaling axis using a monocolonal antibody for CD6. At 19 weeks of age, mice treated with anti-CD6 show improved proteinuria compared to isotype control mice (p< 0.05). MRL/lpr mice develop lymphoproliferative disease which results in abnormally large lymph nodes. Assessing lymphadenopathy at 19 weeks of age, we noted a significant improvement in the anti-CD6 treated mice, compared to the isotype control (p< 0.05). MRL/lpr mice also develop severe skin lesions that have similar pathology to cutaneous lupus. Macroscopic scoring of these lesions showed a significant improvement in the skin disease of the anti-CD6 treated mice compared to the isotype control group (p< 0.05).
Conclusion: Within a spontaneous model of SLE, anti-CD6 treatment ameliorated multiple end organ pathologies, namely in the kidney and skin, while also significantly reducing the lymphoproliferative phenotype of this model. Overall, these results indicate that targeting the CD6-ALCAM pathway may have promising therapeutic potential for multiple end organ pathologies within SLE.
To cite this abstract in AMA style:
Chalmers S, Garcia S, Ayilam Ramachandran R, Mohan C, Ampudia J, Ng C, Connelly S, Putterman C. CD6 Modulation Ameliorates Skin and Kidney Disease in a Spontaneous Murine Model of SLE [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/cd6-modulation-ameliorates-skin-and-kidney-disease-in-a-spontaneous-murine-model-of-sle/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cd6-modulation-ameliorates-skin-and-kidney-disease-in-a-spontaneous-murine-model-of-sle/