Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Dazodalibep (DAZ) is a non-antibody fusion protein that acts as a CD40L antagonist and blocks costimulatory signals between immune cells, including T cells, B cells, and antigen-presenting cells. CD40L inhibition disrupts costimulatory signals that lead to activation of germinal centers (GC), pathogenic B cells, plasma cells, and autoantibodies that are hallmarks of Sjögren’s disease. Among the previously studied biomarkers in Sjögren’s, CXCL13 is produced by activated T Follicular Helper (Tfh) cells and is essential for GC formation and activity.
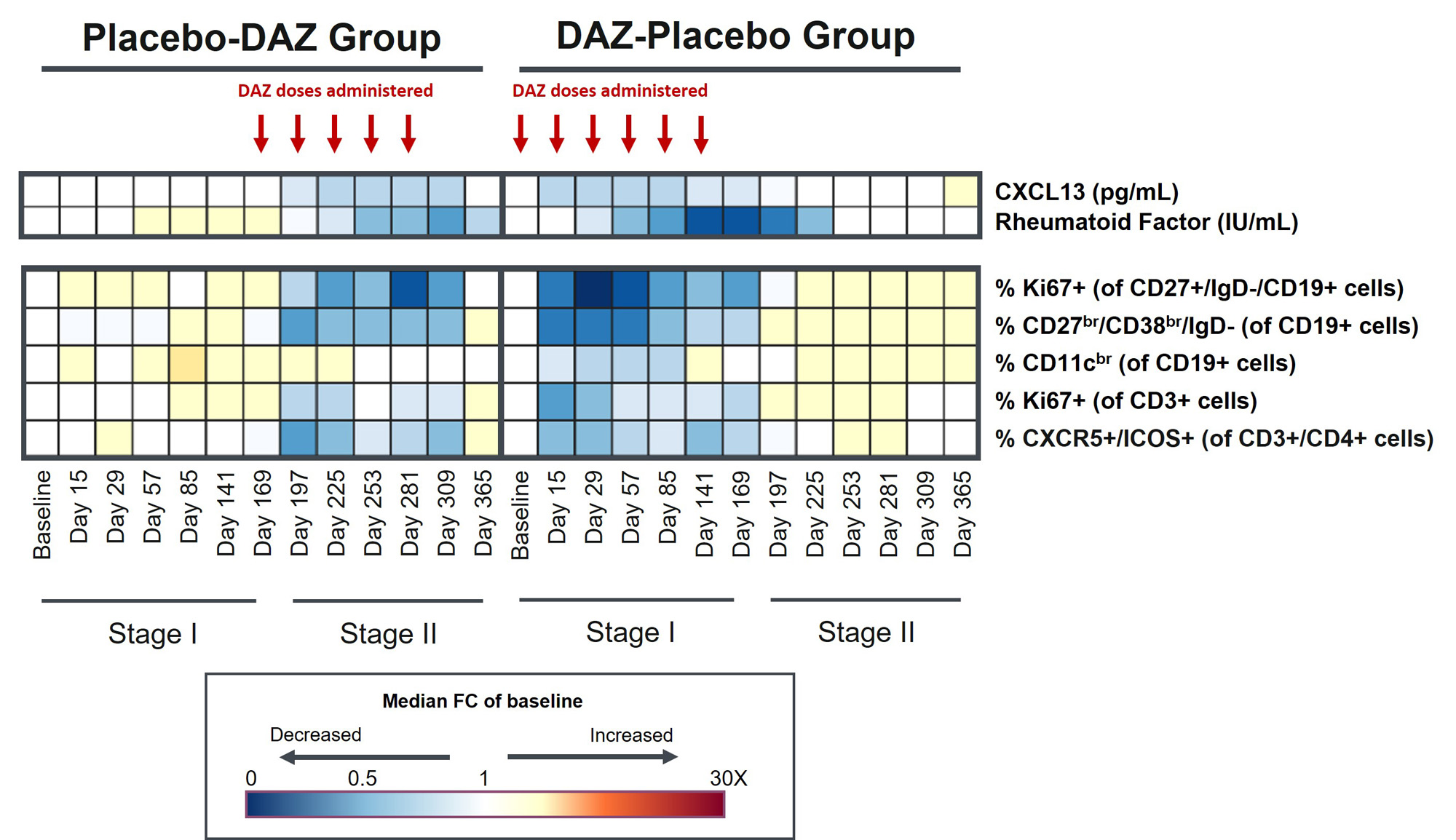
Methods: This study enrolled two distinct populations of subjects with Sjögren’s (NCT04129164). Population 1 (Pop1) included 74 pts with moderate-to-severe systemic disease activity as defined by a EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) ≥ 5. Population 2 (Pop2) consisted of 109 pts with unacceptable symptom burden and limited extraglandular systemic involvement as defined by a EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI) ≥ 5 and ESSDAI < 5. Eligible subjects were randomized 1:1 to receive intravenous DAZ 1500 mg or placebo (PBO) Q2W x 3 doses, then Q4W x 4 additional doses (Stage I). Starting on Day 169, subjects initially randomized to DAZ transitioned to PBO Q4W x 5 doses and those initially randomized to PBO were switched to DAZ Q4W x 5 doses and then followed for 12 weeks (Stage II). B and T cell subsets (Ki67+ post-switch memory B cells [of CD27+/IgD-/CD19+ cells], CD27br/CD38br/Ig D- plasmablasts, CD11cbr atypical memory cells, CXCR5+/ICOS+ TfH, and Ki67+ T cells) were immunophenotyped using flow cytometry. Serum levels of CXCL13 and rheumatoid factor (RF) autoantibodies were assessed by immunoassay and nephelometry, respectively.
Results: Baseline immune profiles of the subjects in Pop1 and Pop2 revealed similar levels of autoantibodies and CXCL13. Baseline levels of CD27br/CD38br/Ig D- plasmablasts, and CD11cbr atypical memory cells were higher in Pop2 than Pop1, while composition of other blood immune cell phenotypes remained similar. In Pop2, compared with the PBO group, DAZ treatment produced significant and rapid reductions from Day 15 onwards in the percentage of Ki67+ post-switch memory B cells, CD27br/CD38br/Ig D- plasmablasts, CD11cbr atypical memory cells, and CXCR5+/ICOS+ TfH cells, as well as decreases in the serum levels of CXCL13 and RF. These results were similar to those previously observed in Pop1 Stage I. In Stage II, when PBO-treated subjects transitioned to DAZ treatment, there were similar sustained reductions in these biomarkers. In DAZ-treated subjects that transitioned to PBO in Stage II, these biomarkers returned to baseline values.
Conclusion: DAZ-mediated CD40-CD40L blockade in subjects with Sjögren’s disease reduced biomarkers by inhibiting T/B cell costimulation and downstream biomarkers of GC formation, activity, and autoantibody production. These findings demonstrate the biological impact of DAZ in subjects with Sjogren’s across populations with either moderate-to-severe systemic disease activity or unacceptable symptom burden.
To cite this abstract in AMA style:
Pham T, Smith M, Mittereder N, Rees W, Alevizos I, St. Clair E, Emson C. CD40L Inhibition with Dazodalibep Rapidly Reduces Blood Biomarkers of T and B Cell Costimulation in Subjects with Sjögren’s Having High Disease Activity or High Symptom Burden [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/cd40l-inhibition-with-dazodalibep-rapidly-reduces-blood-biomarkers-of-t-and-b-cell-costimulation-in-subjects-with-sjogrens-having-high-disease-activity-or-high-symptom-burden/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cd40l-inhibition-with-dazodalibep-rapidly-reduces-blood-biomarkers-of-t-and-b-cell-costimulation-in-subjects-with-sjogrens-having-high-disease-activity-or-high-symptom-burden/