Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid lung disease is a prognostic factors of rheumatoid arthritis in human. The pathogenesis and the mechanism of rheumatoid lung disease is unclear. Zymosan A (ZyA)-treated SKG mice develop not only arthritis but also pneumonitis. To clarify the mechanism of rheumatoid lung disease in human, we analyzed the pneumonitis in SKG mice, which is similar to rheumatoid lung disease in human.
Methods: SKG mice were induced pneumonitis by ZyA injection. The severity of pneumonitis three months after ZyA injection was evaluated by the area of diffusely affected lesion in HE stain, as follows: histological score (HS) 0: affected area <10%, HS1: 10-29%, HS2: 30-59%, HS3: ≥60%. Lung-infiltrating cells (T cells, myeloid cells, innate lymphoid cells (ILCs)) were evaluated by flow cytometry. In vitro, lung-infiltrating cells were cultured with GM-CSF (and IL-4) for 5 days and evaluated by flow cytometry. CSFE-labeled naïve T cells were cultured with isolated CD11b+Gr1dimcells from lung, and stimulated with anti-CD3 and anti-CD28 monoclonal antibodies. After three days, Cell proliferation was examined by measuring CFSE fluorescence with flow cytometry.
Results: Histological analysis revealed that ZyA-treated mice developed various severity of pneumonitis; HS1: 30%, HS2: 50%, HS3: 20%. Unique cells with large and bright nucleus emerged only in the specimen of HS3. Flow cytometric analysis revealed that CD11b+Gr1+cells, CD11b+Gr1dimcells, Th17 cells, regulatory T cells (Treg), and ILC3s were increased, and the proportion of these cells varied depending on the HS: CD11b+Gr1+cells decreased as the progression of HS, while CD11b+ Gr1dimcells emerged only in the lung of HS3 (Figure1). Most of CD11b+ Gr1dimcells expressed CD11c, and the cells facilitated the naïve T cell proliferation in vitro. GM-CSF (and IL-4) induced the differentiation of CD11b+Gr1dimcells from total lung cells. The GM-CSF mRNA was significantly increased in the lung of ZyA-treated SKG mice compared to that of normal saline (NS)-treated SKG mice. Intracellular cytokine staining revealed that GM-CSF-producing Th17 and ILC3s were significantly increased in the lung of ZyA-treated SKG mice.
Conclusion: CD11b+Gr1dimcells, which are induced by GM-CSF produced by Th17 and ILC3s, may facilitate the progression of pneumonitis in SKG mice. 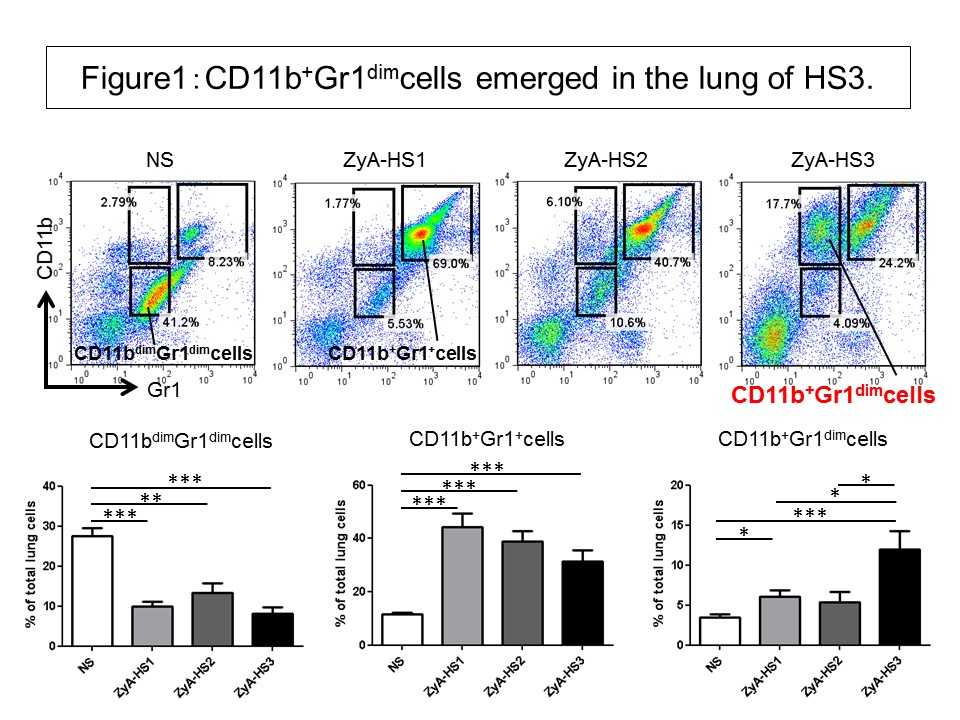
To cite this abstract in AMA style:
Sendo S, Saegusa J, Okano T, Takahashi S, Morinobu A. CD11b+Gr1dimcells, Which Are Induced By GM-CSF Produced By Th17 and Group3 Innate Lymphoid Cell, May Facilitate the Progression of Pneumonitis in SKG Mice [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/cd11bgr1dimcells-which-are-induced-by-gm-csf-produced-by-th17-and-group3-innate-lymphoid-cell-may-facilitate-the-progression-of-pneumonitis-in-skg-mice/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cd11bgr1dimcells-which-are-induced-by-gm-csf-produced-by-th17-and-group3-innate-lymphoid-cell-may-facilitate-the-progression-of-pneumonitis-in-skg-mice/
