Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Progressive pseudo-rheumatoid dysplasia (PPRD) is a rare autosomal recessive disorder characterized by non-inflammatory joint issues that primarily affect the articular cartilage. This leads to progressive joint stiffness and enlargement without accompanying inflammation. Despite its clinical significance, the exact pathogenesis of PPRD remains poorly understood. Our research group previously conducted Exome sequencing on a PPRD pedigree and identified the C223G mutation in CCN6, which is frequently reported in Chinese literature on PPRD. Building on this groundwork, our current study aims to delve deeper into the specific pathways and molecular interactions disrupted by CCN6 mutations in the context of PPRD.
Methods: Exome sequencing was conducted on a PPRD pedigree to pinpoint potential genetic mutations associated with the disorder. To investigate the functional consequences of the identified CCN6 mutation, a lentivirus-based expression vector harboring the mutation was constructed and used to infect chondrocytes, thereby simulating the cellular pathology observed in PPRD. Subsequent analyses included co-immunoprecipitation to study protein interactions, confocal imaging to observe cellular and subcellular structures, Western blotting to assess protein expression levels, and flow cytometry to evaluate changes in cell cycle and apoptosis.
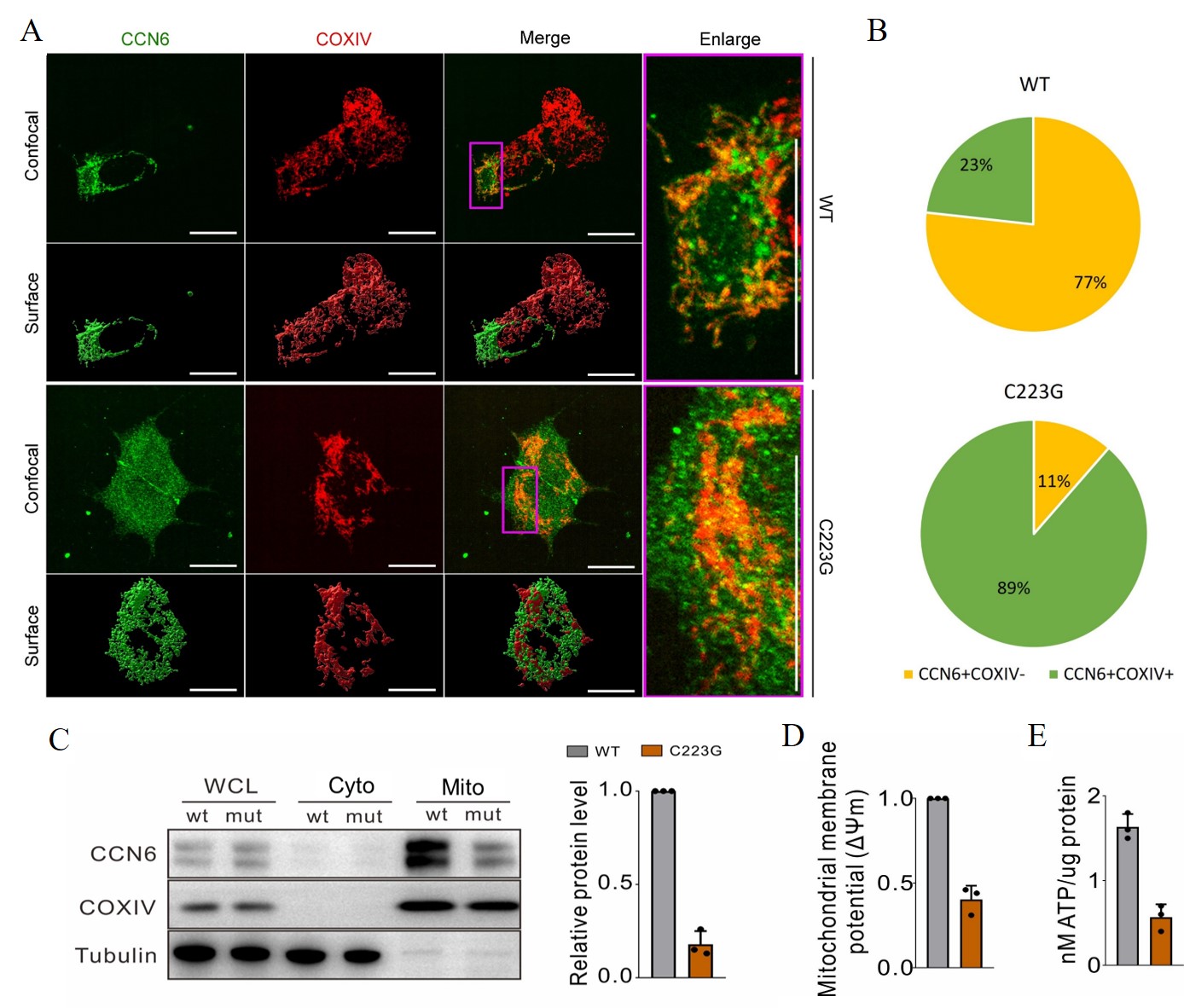
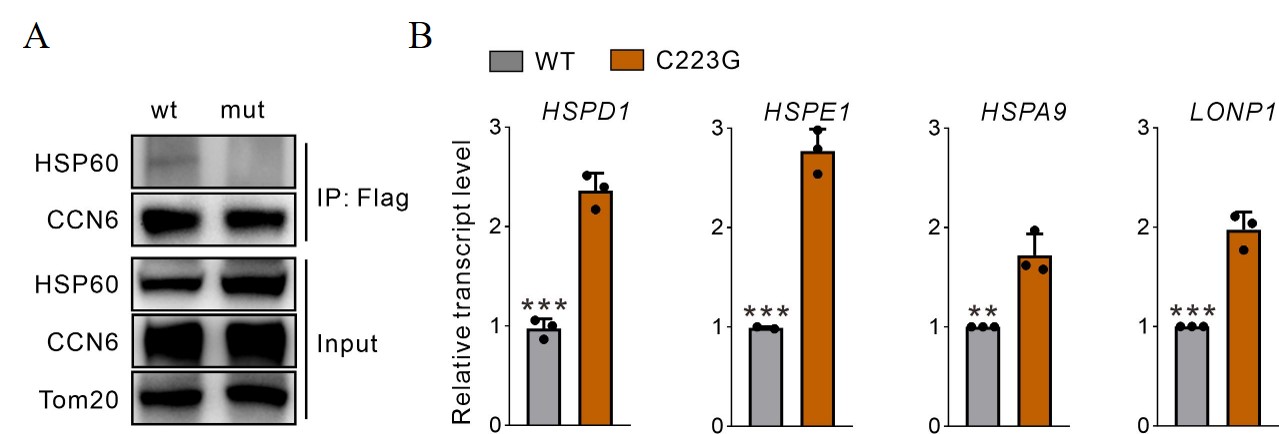
Results: Exome sequencing identified the C223G mutation in the CCN6 gene, which is the most frequently reported mutation in the Chinese literature on PPRD. Subsequent cell transfection experiments showed that wild-type CCN6 localizes to chondrocyte mitochondria, whereas the CCN6 (C223G) mutant remains in the cytoplasm. Further investigations demonstrated that the CCN6-C223G mutation impairs the interaction with the mitochondrial chaperone HSP60, in contrast to the normal binding observed between wild-type CCN6 and HSP60. This impaired interaction leads to abnormal HSP60 function, triggering the activation of the mitochondrial unfolded protein response (mtUPR). The activation of mtUPR disrupts cellular energy metabolism and results in altered gene expression within the nucleus. These findings provide insight into the molecular mechanisms by which the CCN6 mutation contributes to the pathogenesis of PPRD, highlighting the critical role of mitochondrial function and intracellular protein homeostasis in this disorder.
Conclusion: Identifying the CCN6-C223G mutation elucidates its pivotal role in triggering the mitochondrial unfolded protein response (mtUPR) and subsequent dysregulation of chondrocyte function in PPRD patients. These novel insights into the molecular mechanisms underlying PPRD pathogenesis highlight a critical pathway that can be targeted for therapeutic intervention. The findings pave the way for the development of targeted drugs aimed at modulating this specific molecular pathway, offering promising potential for effective treatments for PPRD.
To cite this abstract in AMA style:
Li Y, Bai X, Liu Y. CCN6 Gene Mutation Induces Mitochondrial Dysfunction: The Cause of Progressive Pseudo-rheumatoid Dysplasia [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/ccn6-gene-mutation-induces-mitochondrial-dysfunction-the-cause-of-progressive-pseudo-rheumatoid-dysplasia/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ccn6-gene-mutation-induces-mitochondrial-dysfunction-the-cause-of-progressive-pseudo-rheumatoid-dysplasia/