Background/Purpose: Hydroxychloroquine (HCQ) is a commonly used 1st-line disease-modifying antirheumatic drug (DMARD) for patients with rheumatoid arthritis (RA) in the U.S., while methotrexate (MTX) is the recommended 1st-line DMARD. However, clinical trials of HCQ as a potential therapy for COVID-19 have raised concerns about its cardiovascular safety. We therefore conducted a comprehensive cardiovascular safety evaluation of HCQ treatment compared with MTX treatment in patients with RA.
Methods: Medicare claims data (Parts A/B/D 2008-2016) linked with the National Death Index were used to conduct a cohort study among patients with RA, aged 65 or older, who initiated HCQ or MTX and had not previously used conventional DMARDs. Patients were required to have at least one year of continuous insurance enrollment. The primary outcomes were (1) sudden cardiac arrest or ventricular arrhythmia (SCA/VA), and (2) 3-point major adverse cardiovascular event (MACE). Secondary outcomes were cardiovascular mortality, all-cause mortality, myocardial infarction, stroke, and hospitalized heart failure (HF). A subgroup analysis was performed to investigate the treatment effect modification of history of HF. We performed as-treated analyses using 1:1 propensity score (PS) matching to adjust for baseline confounding. Cox proportional hazards models were fit to estimate hazard ratios (HR) and 95% confidence intervals (CI).
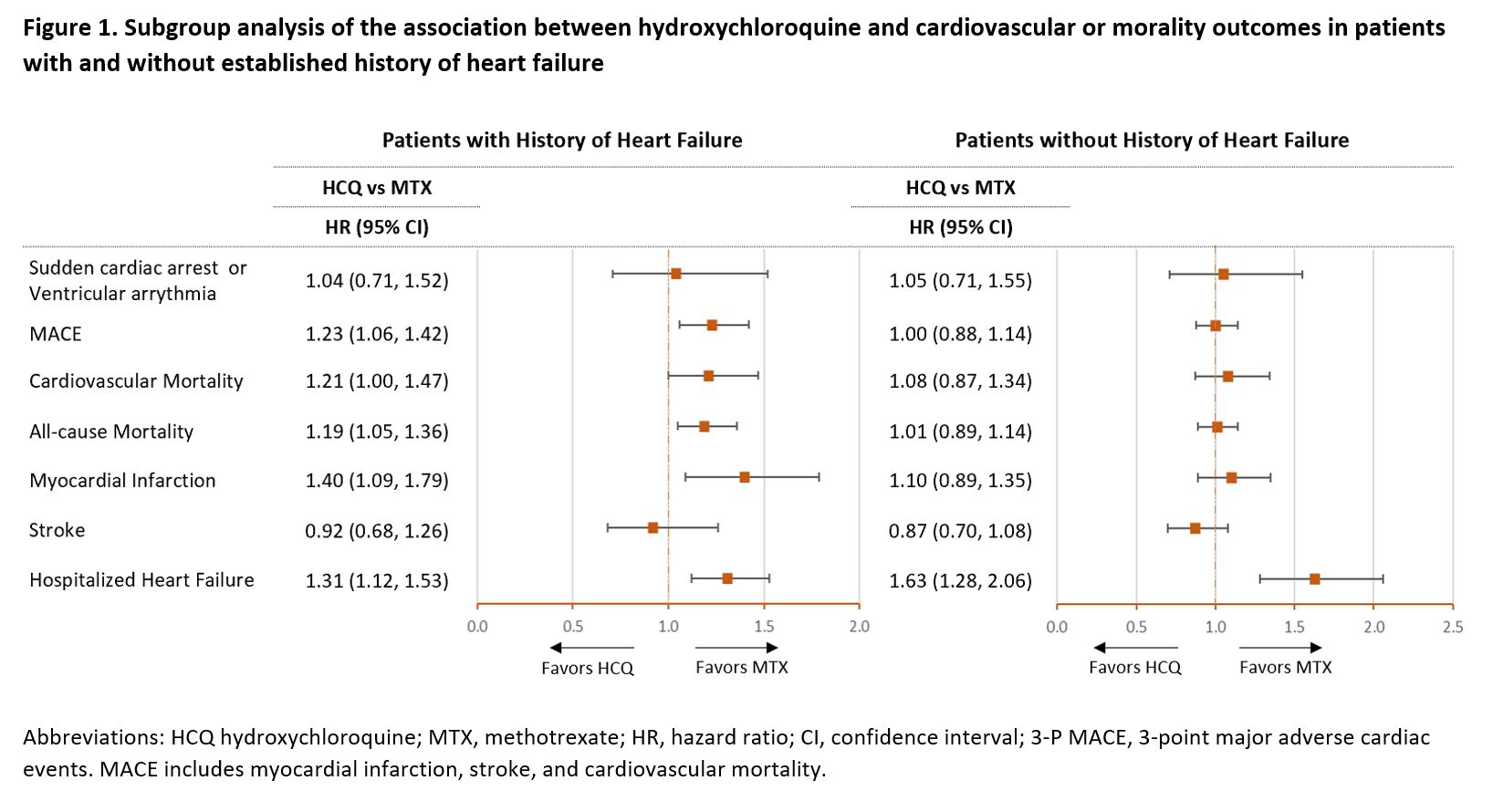
Results: The study included 54,462 PS-matched pairs of RA patients initiating HCQ or MTX. Median duration of follow-up on treatment was 209 days (interquartile range, 103-491 days). In the unmatched cohort, HCQ users were more likely to be female (78.9% vs 73.9%) and less likely to use oral glucocorticoids (65% vs 70.1%) compared to MTX users, all other baseline characteristics were similar between the two groups. After PS matching, the two groups were well balanced on all 59 observed confounders (Table 1). In the primary analysis, HCQ use was not associated with risk of SCA/VA (HR 1.03, 95% CI 0.79-1.35) or MACE (HR 1.07, 95% CI 0.97-1.18) compared with MTX use. In the secondary analyses, HCQ use was associated with increased risks of cardiovascular mortality (HR 1.17, 95% CI 1.02-1.35), all-cause mortality (HR 1.10, 95% CI 1.01-1.20) and hospitalized HF (HR 1.41, 95% CI 1.24-1.61), compared with MTX use (Table 2). Among patients with history of HF, HCQ users had an increased risk of MACE (HR 1.23, 95% CI 1.06-1.42), cardiovascular mortality (HR 1.21, 95% CI 1.00-1.48), all-cause mortality (HR 1.19, 95% CI 1.05-1.36), myocardial infarction (HR 1.40, 95% CI 1.09-1.79) and hospitalized HF (HR, 1.31, 95% CI 1.12-1.53) compared to MTX users. No differences in risks were observed between HCQ and MTX users without history of HF except for an increased risk of hospitalized HF (HR 1.63, 95% CI 1.28-2.06) (Figure 1).
Conclusion: Overall, HCQ treatment has no increased risk of SCA/VA or MACE among RA patients compared with MTX treatment. However, HCQ use appears to be associated with increased risk of MACE, cardiovascular mortality, all-cause mortality, and myocardial infarction compared with MTX use in patients with RA and history of HF. An increased risk of hospitalization for HF was observed in new users of HCQ regardless of prior history of HF.
To cite this abstract in AMA style:
D'ANDREA E, Desai R, He M, Glynn R, Lee H, Weinblatt M, Solomon D, Kim S. Cardiovascular Risk of Hydroxychloroquine in the Treatment of Rheumatoid Arthritis: A Retrospective Cohort Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/cardiovascular-risk-of-hydroxychloroquine-in-the-treatment-of-rheumatoid-arthritis-a-retrospective-cohort-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cardiovascular-risk-of-hydroxychloroquine-in-the-treatment-of-rheumatoid-arthritis-a-retrospective-cohort-study/