Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Filgotinib (FIL) is a Janus kinase (JAK) 1 preferential inhibitor for the treatment of RA. Data from the ORAL Surveillance post-marketing study (NCT02092467) suggest that in patients with active RA aged ≥50 years with ≥ 1 CV risk factor, the risks of cancer and major adverse cardiovascular events (MACE) are higher with the pan-JAK inhibitor tofacitinib vs tumor necrosis factor inhibitors, with higher rates in those aged ≥ 65 vs < 65 years.1
The objective of this analysis was to assess the incidence of malignancies excluding nonmelanoma skin cancer (NMSC), NMSC, MACE and venous thromboembolism (VTE) in patients treated with FIL 200 mg (FIL200) and FIL 100 mg (FIL100) in RA clinical trials.
Methods: Data were pooled from patients treated with FIL200 or FIL100 from DARWIN 1–3 (NCT01888874, NCT01894516, NCT02065700) and FINCH 1–4 (NCT02889796, NCT02873936, NCT02886728, NCT03025308). Data cuts used for the ongoing DARWIN 3 and FINCH 4 studies were May 2, 2022, and May 6, 2022, respectively. Exposure-adjusted incidence rates (EAIRs) per 100 patient-years of exposure (PYE) were calculated for MACE, VTEs, malignancies excluding NMSC, NMSC and treatment-emergent adverse events (TEAEs) leading to death, according to FIL dose (200 vs 100 mg) and age (< 65 vs ≥ 65 years); no statistical testing was performed, so all differences are numerical. MACE and VTE were adjudicated by an independent committee; the cutoff for adjudication was April 3, 2022.
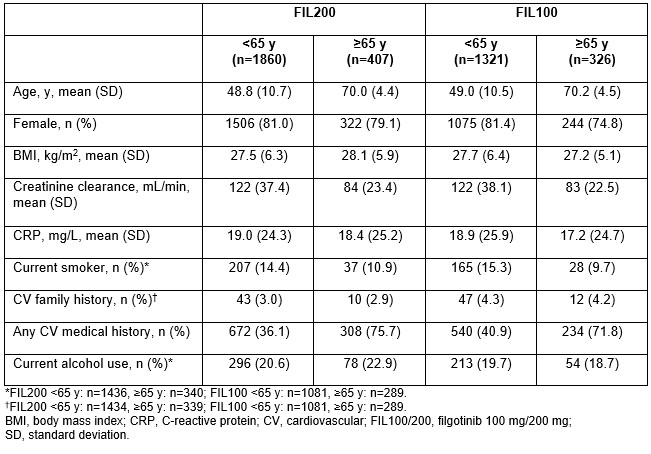
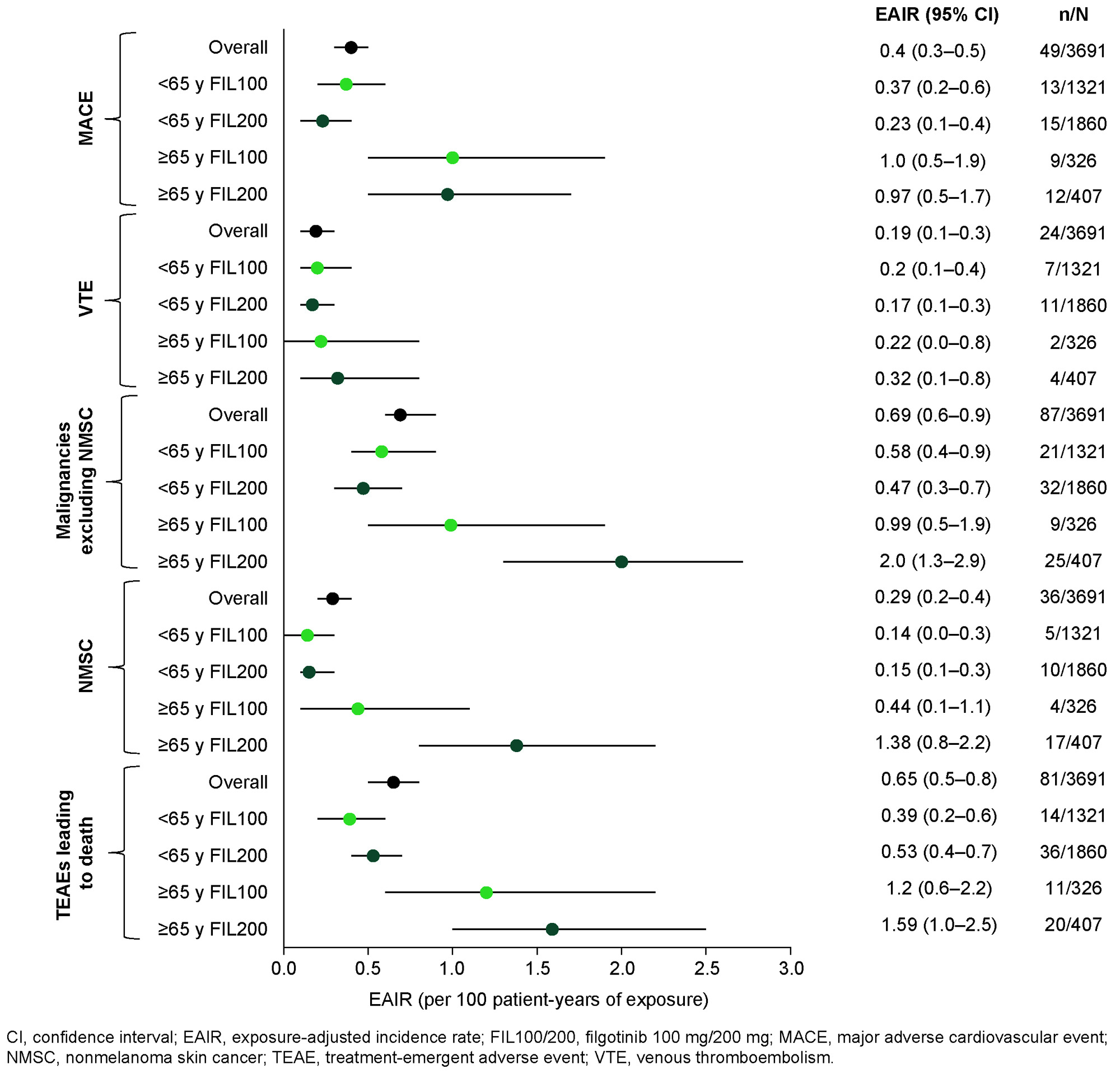
Results: Overall, 3691 patients were treated with FIL for a total of 12,541 PYEs. Median (max) PYE was 3.8 (8.3) years for FIL200 and 3.3 (7.8) years for FIL100. Baseline characteristics are shown in the Table. A greater proportion of those aged ≥ 65 years vs < 65 years had a CV medical history in both the FIL200 (75.7% vs 36.1) and FIL100 (71.8% vs 40.9%) groups. Overall EAIRs (95% confidence interval [CI]) were 0.40 (0.3, 0.5) for MACE, 0.19 (0.1, 0.3) for VTEs, 0.69 (0.6, 0.9) for malignancy excluding NMSC, 0.29 (0.2, 0.4) for NMSC and 0.65 (0.5, 0.8) for TEAEs leading to death. EAIRs of MACE and VTE were higher in patients aged ≥ 65 vs < 65 years but were generally similar for FIL200 and FIL100 within each age group (Figure). EAIRs of malignancies, NMSC and TEAEs leading to death were also higher in the ≥ 65- vs < 65-year group. Within the ≥ 65-year group, EAIRs (95% CI) of these events were numerically higher in the FIL200 vs FIL100 group: 2.0 (1.3, 2.9) vs 0.99 (0.5, 1.9) for malignancies, 1.38 (0.8, 2.2) vs 0.44 (0.1, 1.1) for NMSC, and 1.59 (1.0, 2.5) vs 1.20 (0.6, 2.2) for TEAEs leading to death, respectively.
Conclusion: Rates of MACE and VTE in FIL-treated patients were low and similar for FIL200 and FIL100. There was a higher proportion of patients aged ≥ 65 years vs < 65 years with a CV medical history. In patients aged ≥ 65 years, EAIRs of malignancies, NMSC and TEAEs leading to death were higher with FIL200 vs FIL100, although CIs overlapped.
Reference:
1. Ytterberg SR, et al. N Engl J Med 2022;386:316–26
To cite this abstract in AMA style:
Mariette X, Borchmann S, Aspeslagh S, Calvo- Alén J, Moriggl R, Szekanecz Z, De Leonardis F, Verbruggen N, Van Hoek P, Schmalzing M, Stallmach A, Charles-Schoeman C, Rajendran V, Rudolph C, Watson C, Tanaka Y, Choy E. Cardiovascular (CV) and Malignancy Events in the Filgotinib Rheumatoid Arthritis (RA) Clinical Development Program up to 8.3 Years [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/cardiovascular-cv-and-malignancy-events-in-the-filgotinib-rheumatoid-arthritis-ra-clinical-development-program-up-to-8-3-years/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cardiovascular-cv-and-malignancy-events-in-the-filgotinib-rheumatoid-arthritis-ra-clinical-development-program-up-to-8-3-years/