Session Information
The 2020 Pediatric Rheumatology Symposium, originally scheduled for April 29 – May 2, was postponed due to COVID-19; therefore, abstracts were not presented as scheduled.
Session Type: Poster Breakout Session
Session Time: 5:10PM-5:40PM
Background/Purpose: Localized scleroderma (LS) is a chronic inflammatory and fibrosing disease that causes both cutaneous and extracutaneous (EC) damage. EC involvement (ECI) is common in juvenile LS (jLS), and includes joints, bones, oral cavity, and nervous system. A measure that captures the full spectrum of morbidity would improve disease assessment. The CARRA LS group generated a measure, the Total Morbidity score (TMS), for this purpose. We report on its performance in a prospective cohort of jLS patients beginning methotrexate (MTX) treatment.
Methods: The TMS specifies that items should only be scored if considered secondary to LS, not to treatment or other factor. TMS items were generated based upon literature review and LS expert group discussion. A modular design was used with multiple items scored within each module: cutaneous (i.e., dyspigmentation, subcutaneous atrophy) including extent, musculoskeletal (MSK, i.e., arthritis, contractures), growth difference of body (i.e., limb girth or length difference), head/neuro (i.e., scalp hair loss, facial hemiatrophy, brain involvement), other organ (i.e., vasculopathy). The weighting of scored items was determined by surveys, 1000Minds software, and iterative cycles of testing using case scenarios followed by discussion. We evaluated the TMS in a prospective cohort of jLS patients followed for 12 months after starting MTX treatment (jLS consensus treatment plan pilot study). Clinical assessments included skin scores, physician global assessment of disease damage (PGA-D), and ECI scoring. Data was analyzed by descriptive statistics, with correlations evaluated by Spearman’s rho. p values < 0.05 were considered to be significant.
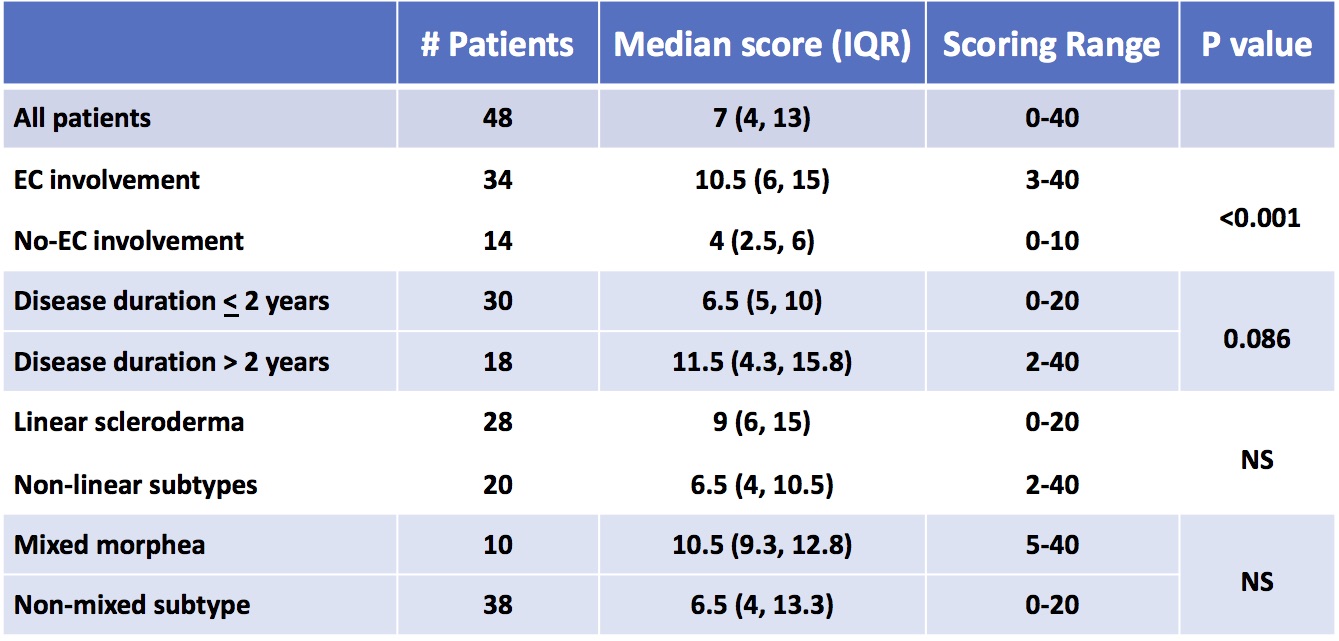
Results: At baseline, 48 patients were scored. There were 33 females, 44 Caucasian, median age of LS onset 9.6 years, and disease duration 12.5 months. The most common LS subtype was linear scleroderma. Baseline median TMS was 7, range 0-40; the 12 months TMS was 6, range 0-35. Patients with ECI had higher scores than those without, p < 0.001, with a trend towards higher TMS in those with disease duration > 2 years, p =0.086 (Table 1). While higher scores were seen in linear scleroderma and mixed morphea subtype patients, differences were not significant.
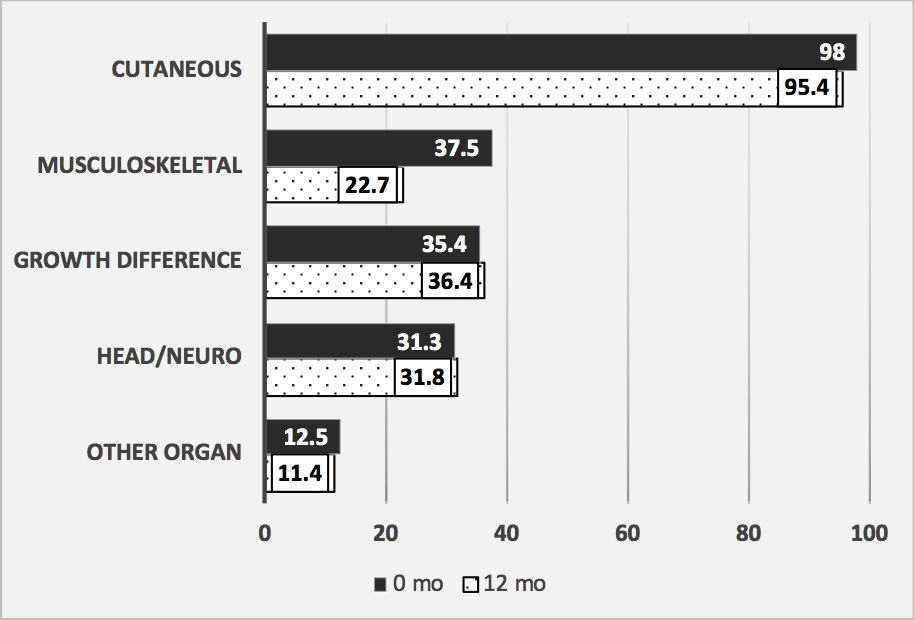
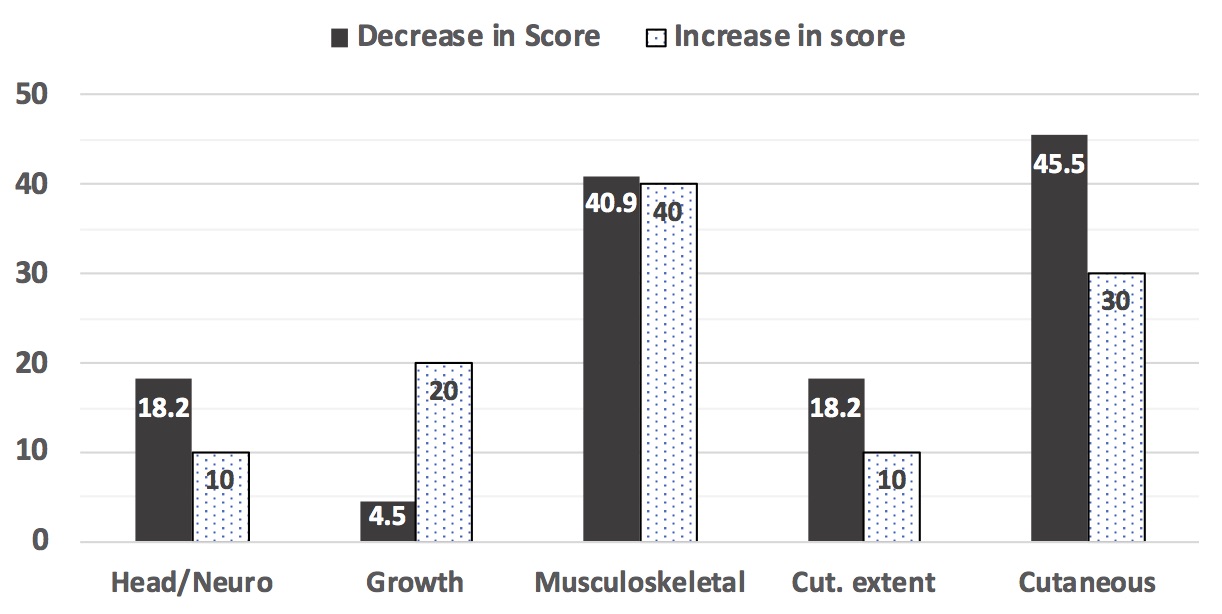
Over 95% of patients had cutaneous damage at 0 and 12 months (Figure 1). At baseline, about 1/3 had MSK, growth differences, and/or head/neuro morbidity, with decline of MSK morbidity at 12 months. Over time, scores declined in 22, increased in 10, and were stable in 12 patients, with change most often occurring in cutaneous and MSK modules (Figure 2). Good correlation was found between TMS and PGA-D at 0 and 12 months (Spearman’s rho 0.778, p < 0.001; 0.756, p < 0.001, respectively). This correlation was better than that found for the localized scleroderma skin damage index (0.629 at 0 months, 0.713 at 12 months).
Conclusion: The Total Morbidity Score was developed to capture the range of, and quantify, LS morbidity. Scores were found to reflect a range of damage levels and morbidities in jLS patients, and vary over time. The TMS showed good correlation with PGA-D. Our findings suggest the TMS could aid tracking of clinical status and response. More study is needed to further assess its performance.
JLS patients beginning methotrexate_based treatment were followed for 12 months. The TMS for 48 patients at the start of treatment (baseline visit) are shown. The number of patient in each category, their median TMS, and scoring range of scores is shown. P_values were calculated by t_test, p< 0.05 considered to be significant
EC: extracutaneous
IQR: interquartile range
JLS patients beginning methotrexate_based treatment were followed for 12 months. The percent of patients that were scored as having a scored morbidity in each of the TMS modules is shown. There were 48 jLS patients at 0 months, 44 at 12 months that were assessed.
Cutaneous refers to skin features (dyspigmentation, dermal atrophy, and skin thickening), subcutaneous tissue atrophy, and extent of these features in affected anatomic sites.
Musculoskeletal (MSK) includes arthritis, joint contractures, myositis, fasciitis. Growth difference includes girth hemiatrophy, limb length differences, and breast, trunk, or buttock hemiatrophy. Head/Neuro includes hair loss, facial disfigurement, seizures, peripheral neuropathy, brain imagining abnormalities, and ocular or oral involvement. Other organ includes gastroesophageal reflux, Raynauds phenomenon, and other miscellaneous LS_related morbidities.
JLS patients beginning methotrexate_based treatment were followed for 12 months. The columns show the percent of patients that had a change in their score of each TMS module from 0 to 12 months. Values for patients that had an increase (10 patients) compared to those that had a decrease (22 patients) in their score are shown separately. Twelve patients had no change in their TMS from 0 to 12 months.
Cut. Extent (cutaneous extent) refers to estimate of surface area of involvement of skin and subcutaneous damage features in each affected anatomic site.
Growth = growth difference module
To cite this abstract in AMA style:
Li S, Patel A, Pope E, Mason T, Sivaraman V, Dedeoglu F, Torok K, Stewart K, Higgins G, Rabinovich C, Fuhlbrigge R, Ibarra M, Hong S, Ferguson P, Becker M, Feldman B, Laxer R. Capturing the Range of Disease Involvement in Localized Scleroderma: The Total Morbidity Score [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 4). https://acrabstracts.org/abstract/capturing-the-range-of-disease-involvement-in-localized-scleroderma-the-total-morbidity-score/. Accessed .« Back to 2020 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/capturing-the-range-of-disease-involvement-in-localized-scleroderma-the-total-morbidity-score/