Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: TRAPS is a rare hereditary autoinflammatory disease characterized by periodic fever and severe systemic and organ inflammation. Successful treatment was achieved with the interleukin-1β inhibitor canakinumab (CAN) in a pivotal phase 3 study, in which 45% of patients achieved clinical remission after 16 weeks (primary endpoint). CAN has been approved for the treatment of TRAPS patients since 2017. The present study investigates the long-term efficacy and safety of CAN under routine clinical practice conditions in pediatric (age ≥2 years) and adult TRAPS patients.
Methods: RELIANCE is a prospective, non-interventional, multicenter observational study in Germany. Patients with a clinically confirmed diagnosis of TRAPS who routinely receive CAN will be enrolled in the study to evaluate the efficacy and safety of CAN under standard clinical conditions at baseline and at six-month intervals.
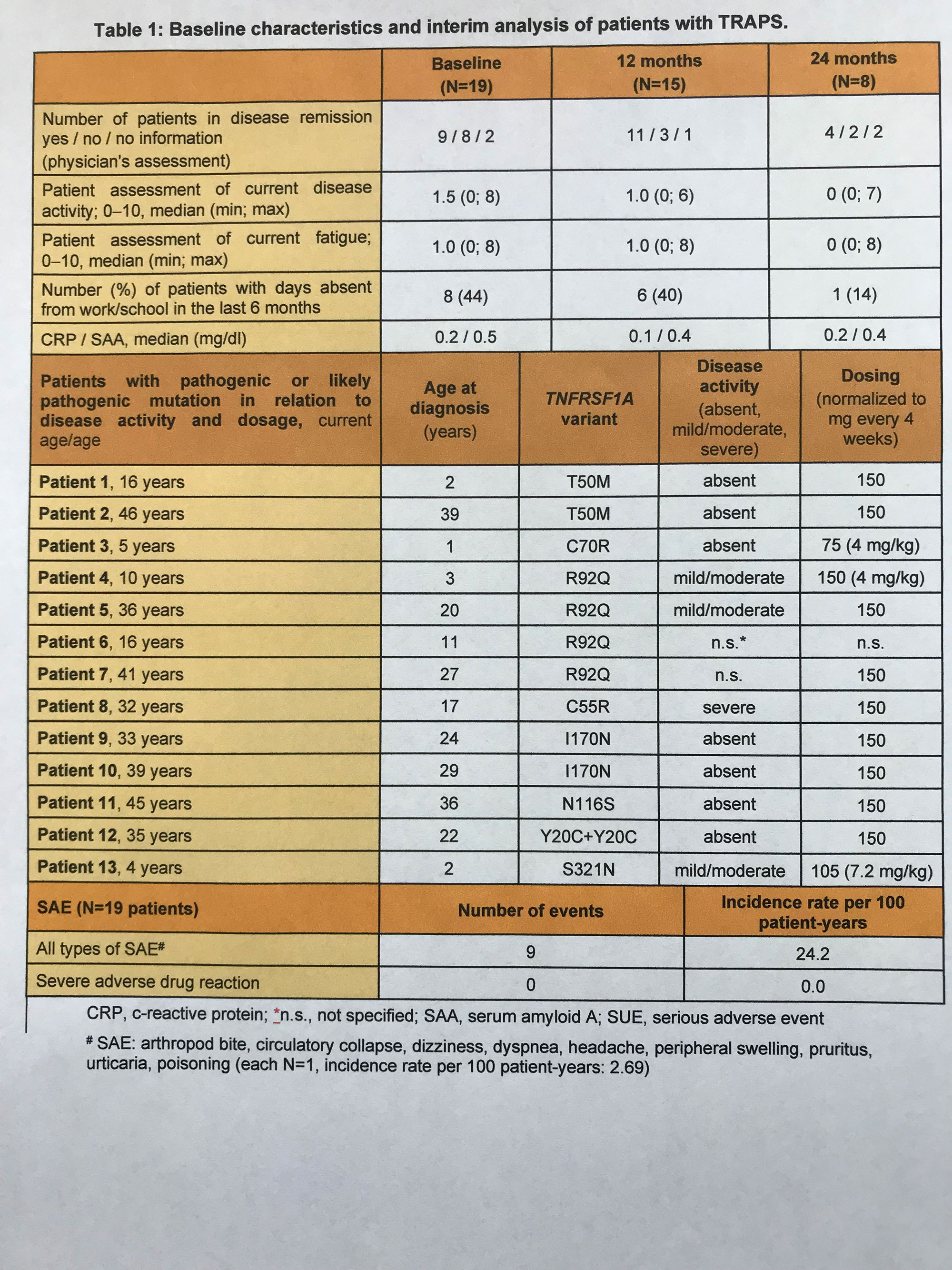
Results: The interim analysis of TRAPS patients enrolled through December 2021 included baseline data (N=19, 1 patient with atypical TRAPS) and preliminary 24-months data. Of these patients, N=12 (63%) were female and the median age at baseline was 16 years (3-43 years), and the median CAN pretreatment time was 1 year (0-4 years).Mutation information was provided for a total of N=13 patients, all of whom were pathogenic or likely pathogenic (Table 1). N=4 patients had dose adjustments (↑ dose increase, ↓ dose reduction): patient 1: ↑, patient 2: ↑↓, patient 3: ↑↑, patient 4: ↑↑↓↑. Preliminary results indicate stable remission according to physician assessment and laboratory parameters. Disease control according to patient assessment showed no significant changes over time (Table 1). Of N=9 SAEs, none occurred in association with therapy. A total of N=7 adverse drug events were observed, all of which were not considered severe.
Conclusion: Baseline and interim data from the RELIANCE trial for TRAPS patients indicate continued good efficacy and safety of various CAN doses.
To cite this abstract in AMA style:
Blank N, Schuetz C, Henes J, Kallinich T, Oommen P, Borte M, Hufnagel M, Janda A, Weber-Arden J, Kuemmerle-Deschner J. Canakinumab in Patients with Tumor Necrosis Factor Receptor-associated Periodic Syndrome (TRAPS) – Long-term Efficacy and Safety Data from a RELIANCE Registry Interim Analysis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/canakinumab-in-patients-with-tumor-necrosis-factor-receptor-associated-periodic-syndrome-traps-long-term-efficacy-and-safety-data-from-a-reliance-registry-interim-analysis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/canakinumab-in-patients-with-tumor-necrosis-factor-receptor-associated-periodic-syndrome-traps-long-term-efficacy-and-safety-data-from-a-reliance-registry-interim-analysis/