Session Information
Date: Monday, October 27, 2025
Title: (1553–1591) Systemic Sclerosis & Related Disorders – Clinical Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Home-monitoring is a suitable strategy to reduce the frequency of hospital visits, and alleviate strain on healthcare resources. However, in systemic sclerosis (SSc) this is challenging because of the heterogenous nature of the disease with regards to multi-organ involvement and trajectories of disease. The aim of this study was to explore the feasibility of using patient reported outcomes measures (PROMs) for home-monitoring by: (1) studying the association between PROMs assessing general health (question 1 and 2 of the 36-Item Short Form Health Survey [SF-36]) and disease progression, and (2) studying the discriminative ability of these PROMs to identify patients with active disease.
Methods: SSc patients fulfilling ACR/EULAR 2013 criteria, with complete SF-36 data, were included from the prospective Leiden CCISS cohort. The first two questions of the SF-36 comprise: “In general, how would you rate your health?” (SF-1) and “Compared to one year ago, how would you rate your health in general?” (SF-2), with possible responses on a 5-point ordinal scale. Disease progression was defined as a worsening of mRSS ≥ 5 and 25%, or ≥ 10% relative decrease in forced vital capacity (FVC), or ≥10% relative decrease in diffusing capacity for carbon monoxide (DLCO), compared to the previous visit). Considering informative follow-up and within-subject correlation we performed mixed-effects logistic regression correcting for age and sex to estimate the association between SF-1 and SF-2 response and disease progression, including time as a fixed effect and random intercept for each patient. A sensitivity analysis was performed in patients who had had three consecutive ‘disease progression free’ – visits, to determine diagnostic test accuracy of SF-1 and SF-2 for disease progression.
Results: In total, 767 SSc patients (n=3186 visits) were identified. Mean age of included patients was 55.2 ± 13.9 years, and 78% was female. Disease progression occurred in 287 patients (n=548 visits; Table 1 and 2). After correcting for age and sex both SF-1 and SF-2 response were not associated with disease progression (very good β -0.2 [-1.4-1.0], good β 0.4 [-0.7 – 1.4], fair β 0.6 [-0.5 – 1.6], poor β 0.7 [-0.4 – 1.8]; and somewhat better β 0.2 [ -0.3 – 0.8], about the same β -0.3 [-0.8 – 0.2], somewhat worse β -0.1 [-0.8 – 0.3], much worse β -0.3 [-0.8 – 0.3]). In total, 174 patients (n=1279 visits) had three consecutive progression-free visits. In this subgroup, disease progression was observed in 26 visits. Both SF-1 and SF2 showed good sensitivity ( >80%) and NPV (≥80%) to identify the patients with progression (Table 3).
Conclusion: Patient reported general health and change in health, as assessed with SF-36, are not associated with disease progression in the general SSc population, and are therefore not helpful for home-monitoring purposes for this population. However, when selecting a subgroup of patients with stable and mild disease for ≥ 3 years, general PROMs show good sensitivity and negative predictive value to identify patients with stable disease. We conclude that general PROMs hold potential for implementation in home-monitoring of SSc patients with mild disease.
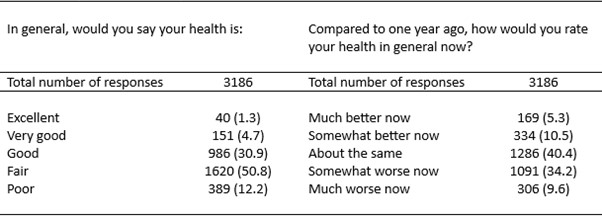 Table 1. Baseline characteristics of SSc patients in the CCISS cohort.
Table 1. Baseline characteristics of SSc patients in the CCISS cohort.
.jpg) Table 2. Responses to SF-1 and SF-2 in the CCISS cohort.
Table 2. Responses to SF-1 and SF-2 in the CCISS cohort.
.jpg) Table 3. Diagnostic test accuracy of patient reported health in stable SSc.
Table 3. Diagnostic test accuracy of patient reported health in stable SSc.
To cite this abstract in AMA style:
Hoekstra E, van der Wouden K, Madari Q, Ahmed S, Kwant L, Hortensius-Varkevisser A, Marges E, Ciaffi J, Schouffoer A, Huizinga T, de Vries-Bouwstra J. Can We Use Patient Reported Outcomes For Home-monitoring in SSc? [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/can-we-use-patient-reported-outcomes-for-home-monitoring-in-ssc/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/can-we-use-patient-reported-outcomes-for-home-monitoring-in-ssc/
