Session Information
Date: Sunday, November 8, 2020
Session Type: Abstract Session
Session Time: 10:00AM-10:50AM
Background/Purpose: Rheumatoid Arthritis (RA) is a chronic disease that involves frequent patient-provider interaction and self-monitoring, both integral to successful disease management. We developed a novel mobile health smartphone app with a voice-enabled feature to help patients virtually track disease activity and ask general questions about RA. A user-centered design-based approach was used to develop and test the app. This abstract will present information from several phases of development and sources of information including: exit interviews from the previous (V1.0) trial, focus groups, adherence with a revised app (V2.0), outcome data (V2.0), and satisfaction surveys (V2.0).
Methods: We recruited patients with RA who participated in a prior trial of the same app (V1.0). They were asked to help design and test V2.0. We conducted exit interviews from the prior trial and used these results to develop a new prototype of V2.0 that included voice enablement and a question & answer (Q&A) function. The prototype was tested in two focus groups of patients (N=8) and one with providers (N=4). Based on focus group feedback, the V2.0 app was built and given to 26 patients to test for 60 days. The V2.0 app asks patients to fill in patient reported outcomes (PROs) daily; PROs rotate between disease activity, mood, fatigue, sleep, function and pain. After the trial, subjects completed satisfaction surveys to provide feedback on their experience. Adherence to PROs was defined as questions answered over total daily app questions.
Results: The exit interviews with V1.0 trial participants revealed several priorities for V2.0 including voice enablement and Q&A function. Focus groups showed the majority of participants found these functions helpful and also helped refine the Q&A library. These RA patients made suggestions regarding diction, speed and options embedded within the voice assistant. The 26 V2.0 trial patients were 77% female and 35% were aged 45-54 years. Interim analyses of adherence to daily PROs during the first 28-day period was 58% (95% CI ± 4.2) (Figure 1). Less than 1% of PROs were completed with the voice-enabled feature. Disease activity, measured twice weekly by the RADAI-5, was stable over four weeks, remaining within the moderate disease activity range (3.2 to 5.4) (Figure 2). Of the 20 patients who completed a satisfaction survey after a full 60-day trial, 85% of subjects were satisfied with their experience (Table). It appeared that only half of the patients had used the voice assistant; not all were satisfied with the voice assistant. The voice assistant had an overall 49% success rate at understanding and answering a user’s request. Most questions focused on simple medication issues (dosage, interactions, time of day, etc).
Conclusion: We developed a novel mobile health PRO app (V2.0) with a voice assistant employing a user-centered design and conducted preliminary user testing. While adherence was moderate and disease activity was reported as stable, the voice assistant was not tried by all patients limiting feedback. While the Q&A library was widely used, not all patient questions could be answered and some participants were dissatisfied. Exit interviews will further examine patient experience to plan for the next iteration.
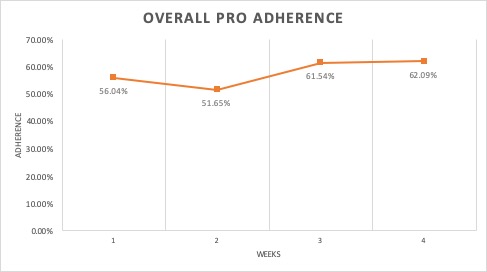 Figure 1: Overall adherence during a 4-week period with daily PRO surveys on disease activity, mood, fatigue, sleep, function and pain.
Figure 1: Overall adherence during a 4-week period with daily PRO surveys on disease activity, mood, fatigue, sleep, function and pain.
 Figure 2: Results from the RADAI-5 PRO surveys, disseminated twice weekly, over a 4-week period with median values and interquartile ranges.
Figure 2: Results from the RADAI-5 PRO surveys, disseminated twice weekly, over a 4-week period with median values and interquartile ranges.
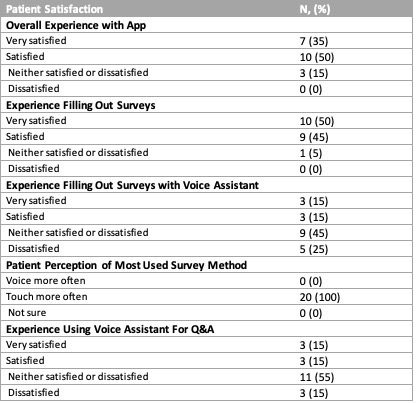 Table: Patient satisfaction survey results after a 60-day trial testing the V2.0 app (N=20).
Table: Patient satisfaction survey results after a 60-day trial testing the V2.0 app (N=20).
To cite this abstract in AMA style:
Murray M, Penney S, Landman A, Elias J, Richard E, Hartog B, Solomon D. “Can I Help You with Your RA Today?”: A Pilot Study on the User Experience with a Voice-Enabled Smartphone App to Virtually Monitor Disease Activity and Collect ePROs in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/can-i-help-you-with-your-ra-today-a-pilot-study-on-the-user-experience-with-a-voice-enabled-smartphone-app-to-virtually-monitor-disease-activity-and-collect-epros-in-rheumatoid-art/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/can-i-help-you-with-your-ra-today-a-pilot-study-on-the-user-experience-with-a-voice-enabled-smartphone-app-to-virtually-monitor-disease-activity-and-collect-epros-in-rheumatoid-art/
