Session Information
Date: Sunday, November 17, 2024
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Clinically isolated aortitis (CIA) is aortitis in patients without evidence of systemic disease, infection, or involvement of other vascular territories. CIA is discovered incidentally in up to 12 % of thoracic aortic aneurysm surgical repairs. Understanding of CIA’s pathogenesis remains limited, complicating diagnosis and management. Neutrophil extracellular traps (NET) have been implicated in escalating inflammation and vasculopathy in various diseases, including non-inflammatory aortic aneurysms. While elevated levels of circulating NET and impaired NET degradation have been observed in other large vessel vasculitides like giant cell arteritis (GCA) and Takayasu arteritis (TAK), the role of NET in CIA has not been studied. Our study aimed to evaluate multiple circulating NET markers in CIA patients and determine their potential role in promoting endothelial damage in aortitis.
Methods: Utilizing the MI-Aorta Aortitis Cohort, we identified 15 CIA patients diagnosed by aortic pathology or advanced imaging studies. Circulating NET markers and endothelial activation markers (ICAM-1, VCAM-1) were measured in plasma from patients with CIA, TAK (n=7), and GCA (n=11), as well as patients with thoracic aneurysms without aortitis (n=6). Additionally, we conducted ex vivo and in vitro experiments exposing human aortic endothelial cells (HAoEC) to CIA patient plasma or recombinant calprotectin to assess potential impacts on endothelial function.
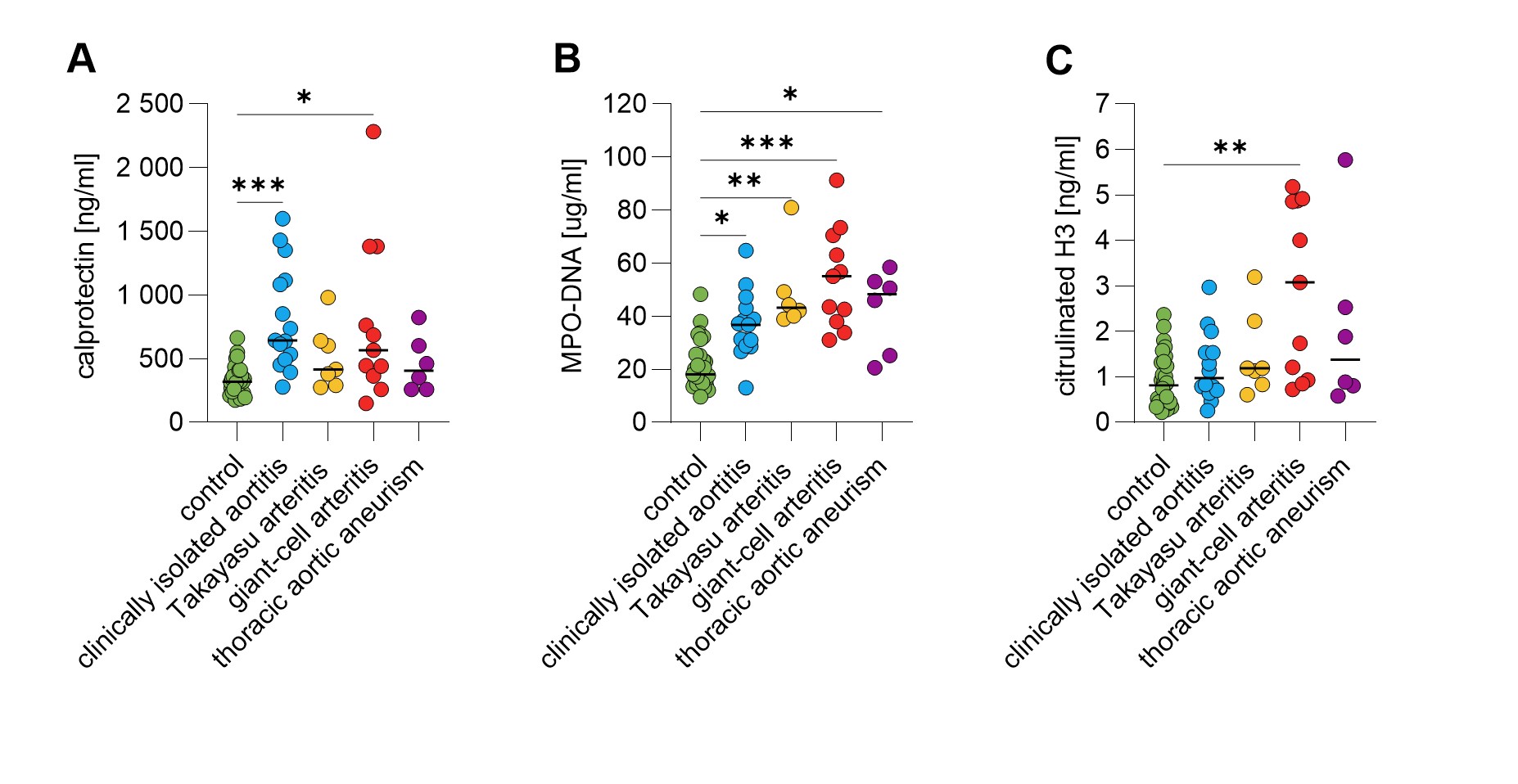
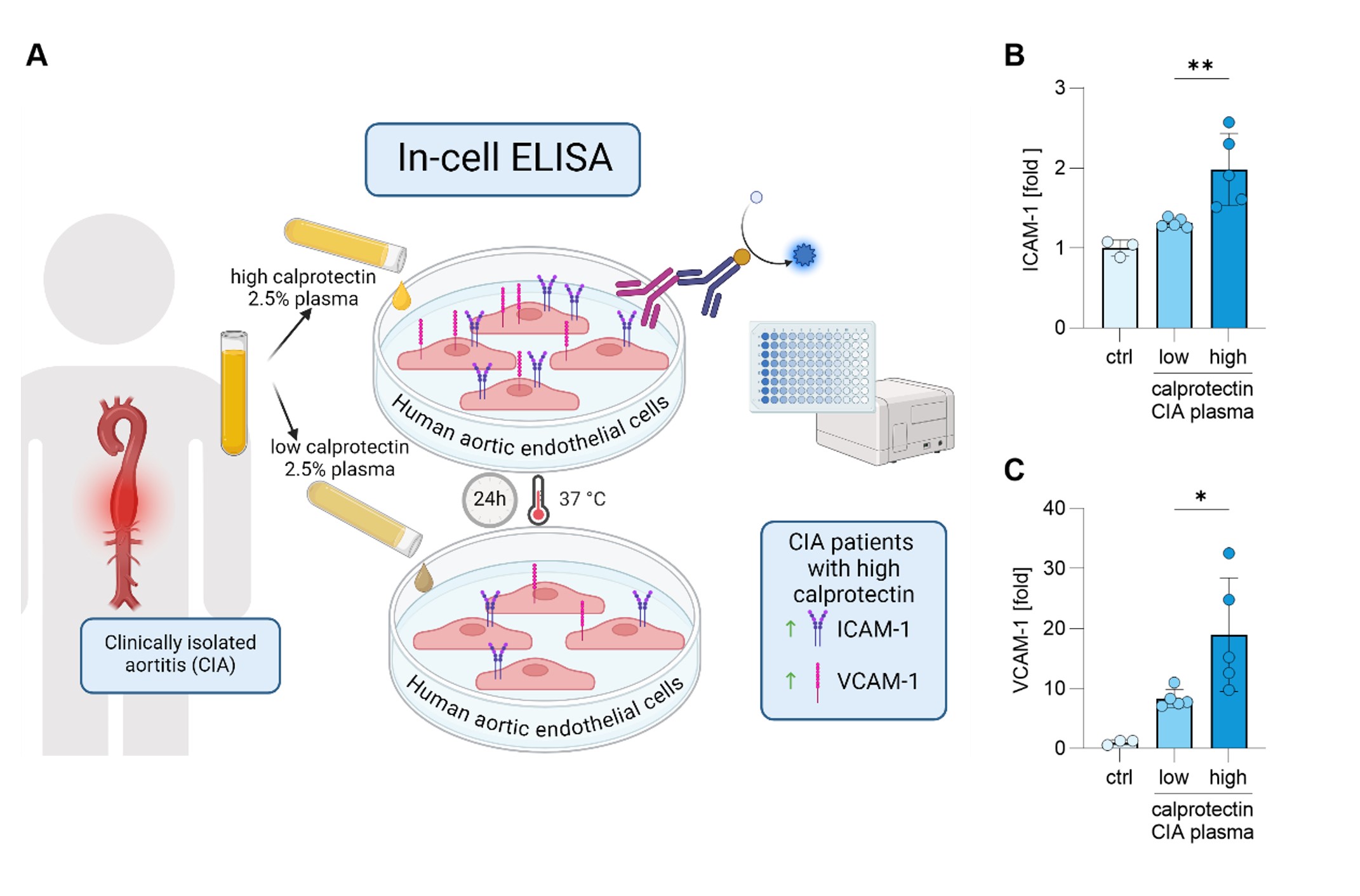
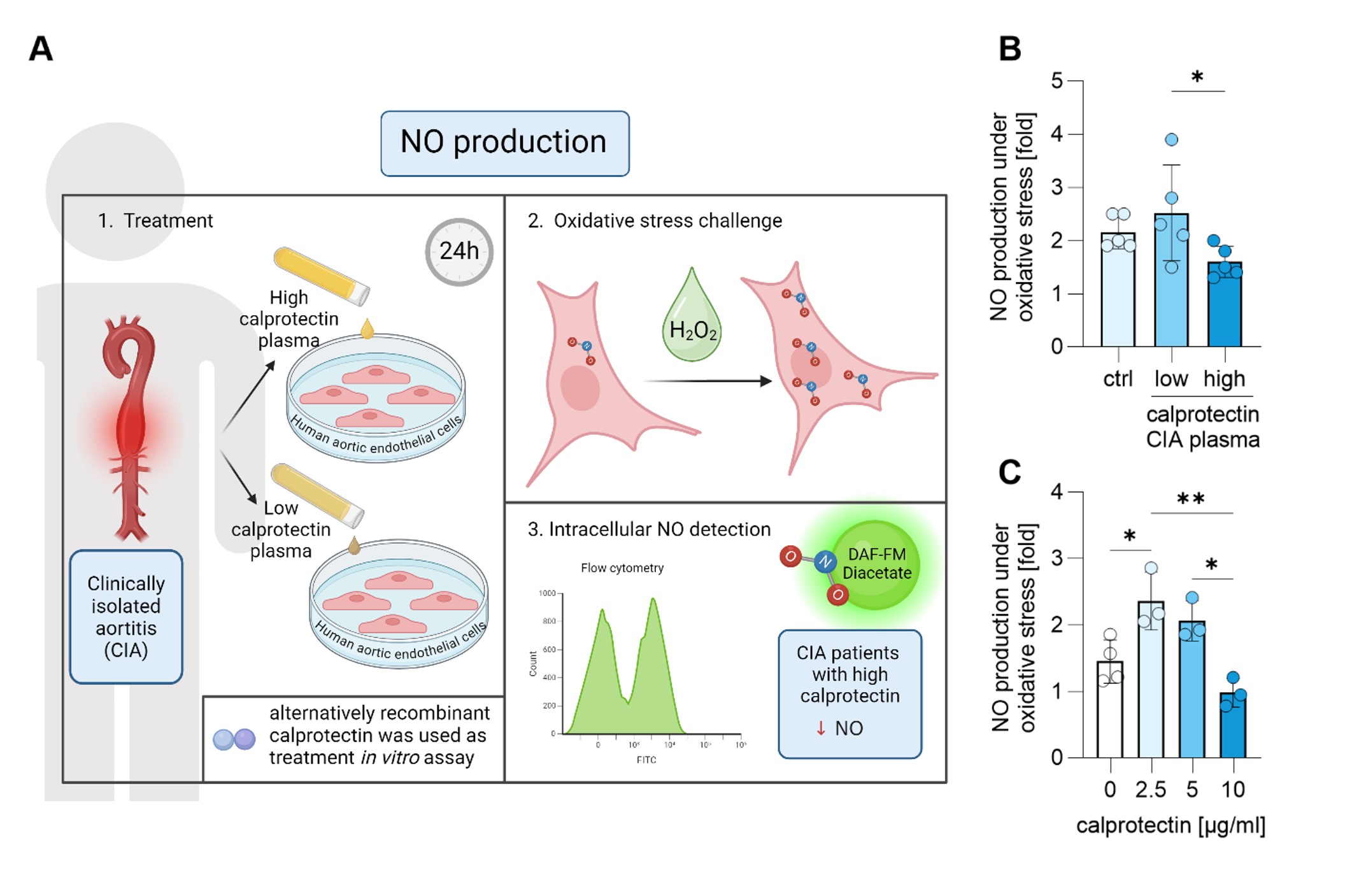
Results: Among the circulating NET markers evaluated, calprotectin levels were elevated in both CIA and GCA plasma (Fig 1A). Increased levels of myeloperoxidase-DNA complexes were observed in all conditions (Fig 1B). Citrullinated histone H3 was uniquely elevated in GCA patients (Fig 1C). Additionally, CIA patients exhibited elevated levels of circulating ICAM-1 and VCAM-1 (p< 0.05, p< 0.001), whereas GCA patients only showed elevated VCAM-1 (p< 0.05). Calprotectin in CIA patients strongly correlated with CRP levels (r=0.889, p< 0.001). Mechanistically, high-calprotectin CIA plasma activated cultured HAoECs, leading to increased expression of surface adhesion molecules ICAM-1 and VCAM-1, crucial to inflammation and vasculopathy (Fig 2B-C). Furthermore, compared to CIA plasma with low calprotectin levels, CIA plasma with high calprotectin levels impaired the ability of HAoECs to produce nitric oxide (NO) under oxidative stress (Fig 3B), a capacity vital for maintaining vascular tone and mitigating inflammation following injury. Treating HAoECs with a high dose of recombinant calprotectin also compromised NO production under oxidative stress (Fig 3C).
Conclusion: Our study identifies calprotectin as a significant circulating NET marker elevated in CIA patients, where it correlates with other inflammatory indices. Mechanistically, calprotectin impairs endothelial integrity and reduces intracellular NO production. These findings suggest a mechanism by which calprotectin may contribute to endothelial dysfunction in CIA. In summary, calprotectin is a mechanism-informed biomarker that warrants further investigation in this challenging clinical scenario.
Plasma samples were collected from 15 patients with clinically isolated aortitis, 7 with Takayasu arteritis, 11 with giant-cell arteritis, 6 with thoracic aneurysm without aortitis, and 34 healthy controls. Circulating levels of (A) calprotectin, (B) MPO-DNA complexes, and (C) citrullinated histone H3 were measured in patients’ plasma. Statistical analyses were performed using the Kruskal-Wallis test with multiple comparisons. * – p<0.05; ** - p<0.01; *** - p<0.001; MPO-DNA - myeloperoxidase-DNA complexes
(A) Schematic of a novel in-cell ELISA for HAoEC. HAoEC were cultured for 24h with 2.5% plasma from CIA patients with high or low calprotectin levels. Cells were then fixed, and surface expression of (B) ICAM_1 and (C) VCAM_1 was quantified as absorbance, using HRP coupled antibody. Results are presented as relative changes to unstimulated cells. Statistical analyses were performed using the Kruskal-Wallis test with multiple comparisons. * – p<0.05; ** - p<0.01
(A) Schematics of experiments. HAoECs were treated with 10% plasma from healthy controls and patients with high and low calprotectin for 24h. Cells were stained with DAF-FM Diacetate to analyze intracellular nitric oxide (NO) production via flow cytometry. Cells were challenged with hydrogen peroxide (H2O2) to assess their NO production capacity, and the ratio of NO produced after oxidative stress (H2O2) to baseline NO production was evaluated. (B) CIA plasma with high calprotectin levels impaired the HAoECs’ ability to produce NO in response to oxidative stress compared to plasma with low calprotectin levels. (C) Similarly, a high dose of recombinant calprotectin also impaired HAoECs’ NO production under oxidative stress. Statistical analyses were performed using the Kruskal-Wallis test with multiple comparisons. * – p<0.05; ** - p<0.01; ctrl – healthy control
To cite this abstract in AMA style:
Kmetova K, Chong E, Somanathapura K N, Sugur K, Kluge L, Yalavarthi S, Ford J, Singer O, Zuo Y. Calprotectin, a Mechanistically Informed Biomarker for Clinically Isolated Aortitis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/calprotectin-a-mechanistically-informed-biomarker-for-clinically-isolated-aortitis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/calprotectin-a-mechanistically-informed-biomarker-for-clinically-isolated-aortitis/