Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Sjögren’s disease (SjD) is a systemic autoimmune disease in which interferons (IFNs) are believed to play a major role/ JAK inhibitors (JAKinibs) block IFN activation pathways; yet little is known about the effect of JAK inhibitors on SjD mouse models. Furthermore, although animal models are invaluable tools to mimic complexities of human disease, little is known about how certain commonly used congenic SjD mice compare to control strains. Our objective was to compare congenic and control SjD model features and to determine the effect of the JAK inhibitor, ruxolitinib (ruxo), on SjD in mouse models.
Methods: We treated females from two SjD mouse models (NOD.B10Sn-H2b/J [NOD.B10] and C57BL/6.NOD-Aec1Aec2 [Aec]) with ruxo 60 mg/kg twice daily or vehicle from 24 to 26 weeks. Stimulated salivary flow (pilocarpine) at the end of two weeks and at baseline. We obtained blood and salivary gland tissue for analyses of glandular and peripheral immune dysfunction. We performed IgG and IgM ELISA per manufacturer specifications. We performed focus score (number of infiltrates comprising 50 or more mononuclear cells per four mm2) or percent infiltrate (the area of mononuclear cell infiltrate/total gland area). Statistical analyses include ANOVA or unpaired student’s t-tests.
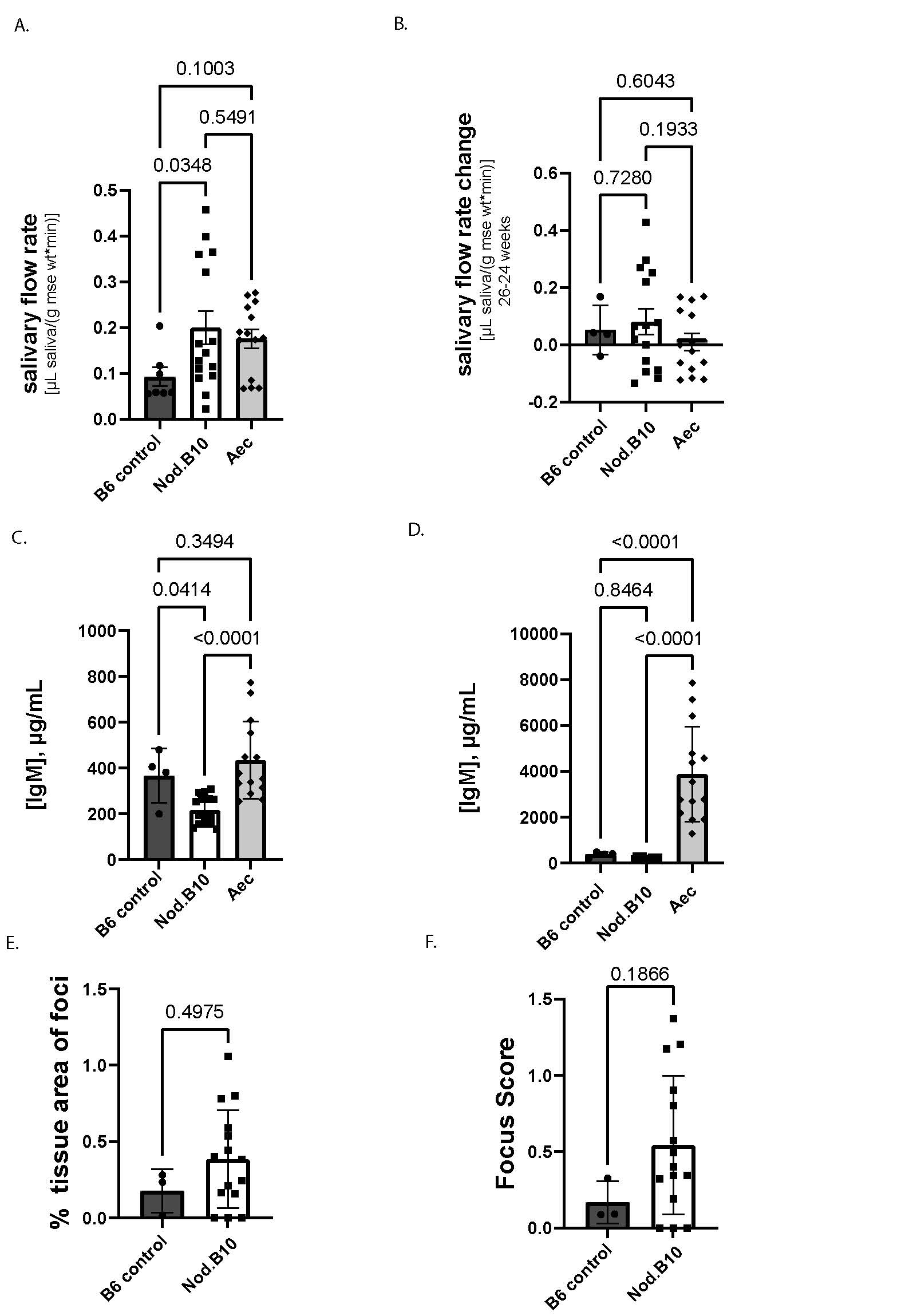
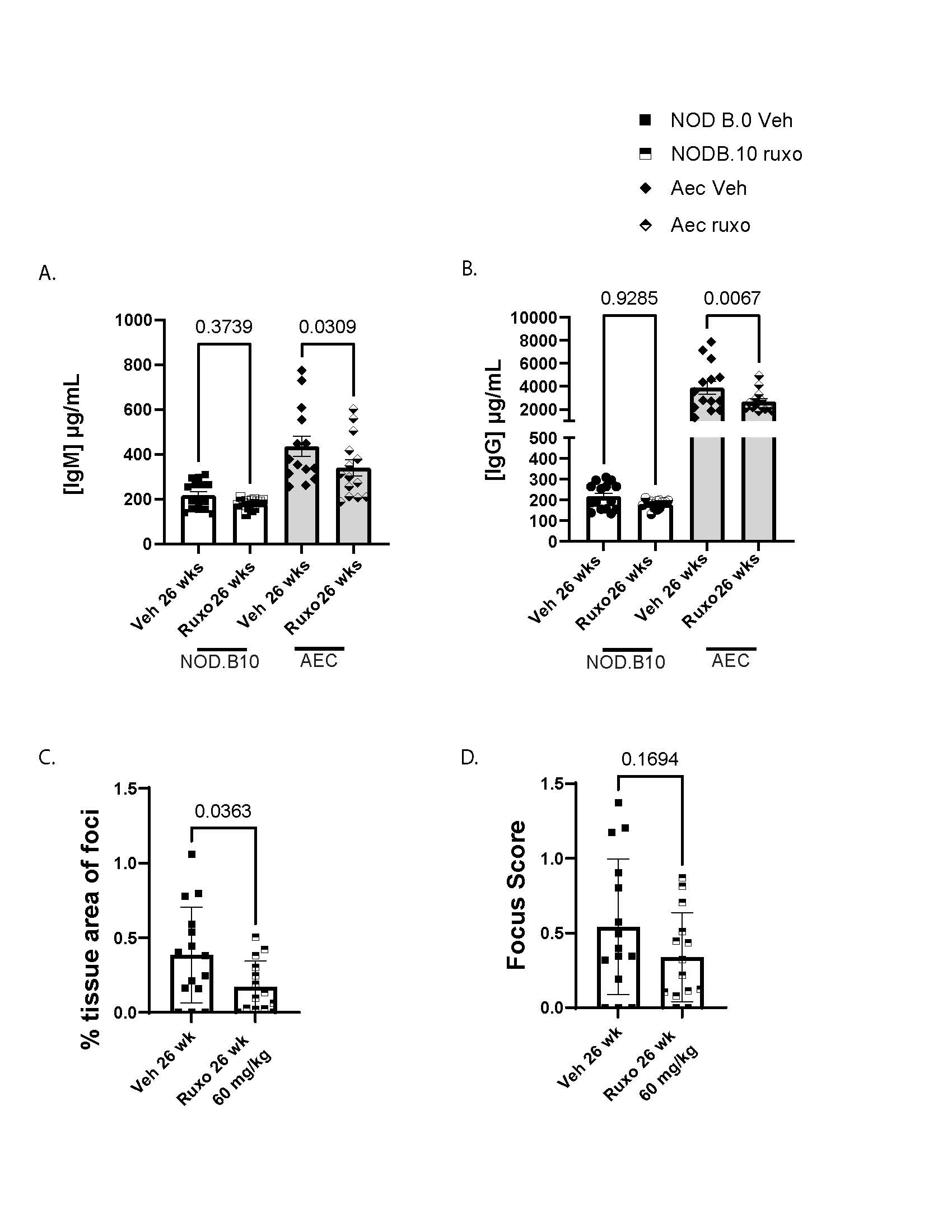
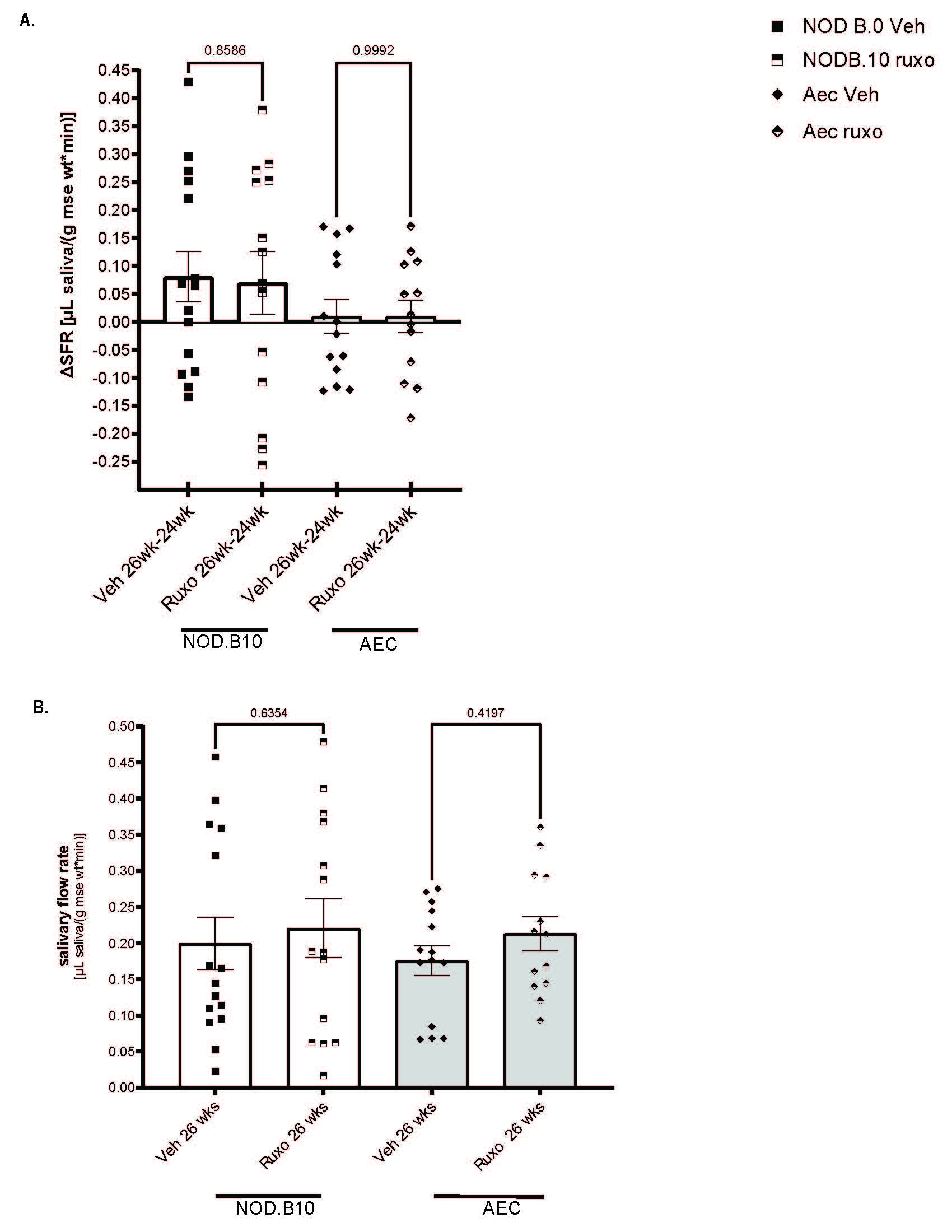
Results: NOD.10 mice make more saliva than B6 controls and stimulated saliva does not differ between mice over two weeks (Fig 1A-B). IgM production is higher in Aec mice than Nod.B10 mice (Fig 1C) and IgG is higher in Aec than both B6 controls and NOD.B10 mice (Fig 1D). NOD.B10 mice have a trend toward greater lymphocytic infiltrate than B6 control mice, but this did not achieve statistical significance (Fig 1E-F). We determined that IgM and IgG improved after two weeks of ruxo therapy in Aec mice (Fig 2A-B). We also found that inflammatory cell infiltrate improved with ruxo therapy in NOD.B10 mice (Fig 2C-D). We found no difference in the amount of saliva produced by mice after two weeks of ruxo treatment (Fig 3A-B).
Conclusion: Aec mice have a more robust SjD hypergammaglobulinemia phenotype than NOD.B10 SjD mouse models so might be a preferred model for testing B-cell targeting drugs. Ruxo seems to improve this immune dysfunctionality in SjD mouse models, but does not improve salivary flow. Future directions include completion of mouse phenotyping and single cell RNA seq to disentangle the mechanisms driving therapeutic response features that differ between responder and non-responder mice.
To cite this abstract in AMA style:
McCoy S, Gurevic I. C57BL/6.NOD-Aec1Aec2 Mice Recapitulate Sjögren’s Serology Better Than NOD.B10Sn-H2b Models and JAK Inhibitor Treatment Improves Immunoglobulins and Salivary Gland Inflammation but Not Salivary Flow in Sjögren’s Mice [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/c57bl-6-nod-aec1aec2-mice-recapitulate-sjogrens-serology-better-than-nod-b10sn-h2b-models-and-jak-inhibitor-treatment-improves-immunoglobulins-and-salivary-gland-inflammation-but-not-salivary/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/c57bl-6-nod-aec1aec2-mice-recapitulate-sjogrens-serology-better-than-nod-b10sn-h2b-models-and-jak-inhibitor-treatment-improves-immunoglobulins-and-salivary-gland-inflammation-but-not-salivary/