Session Information
Date: Saturday, November 16, 2024
Title: Abstracts: T Cell Biology & Targets in Autoimmune & Inflammatory Disease
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: B cells are pivotal in autoimmune disease pathogenesis as they produce autoantibodies and undergo aberrant maturation processes, and B-cell dysregulation and the production of autoreactive long-lived plasma cells (LLPCs) have been identified as a possible mechanism in chronic disease. Recently anti-CD19 and anti-BCMA Chimeric Antigen Receptor T cells (CAR-T) treatment, which targets B cells and plasma cells, have shown promising early clinical results in the treating various autoimmune diseases. This suggests the importance to targeting both B cells and plasma cells to eliminate pathogenic autoantibodies. However, there is debate about whether CD19 is the most appropriate B cell target. Evidence from B cell malignancies suggests that CD20 CAR-T might result in better survival in diffuse large B-cell lymphoma (DLBCL) than CD19 CAR-T and could even be effective in patients relapsing after CD19 CAR-T therapy. Therefore, we have developed C-CAR168, a novel fully human CD20/BCMA bi-specific autologous CAR-T cell product, for the treatment of autoimmune diseases.
Methods: Cell-based function assays, including cytokine release and cell killing, were used to determine the specificity and reactivity of C-CAR168, and we performed in vivo function studies using immunodeficient mouse xenograft models. A whole genome human membrane proteome array and healthy human tissue immunohistochemistry assays using a rabbit Fc conjugated scFv of C-CAR168 were performed to evaluate the off-tumor toxicity. Potential carcinogenicity risks of C-CAR168 were examined using IL-2 independent growth in vitro and colony formation in the soft-agar assays.
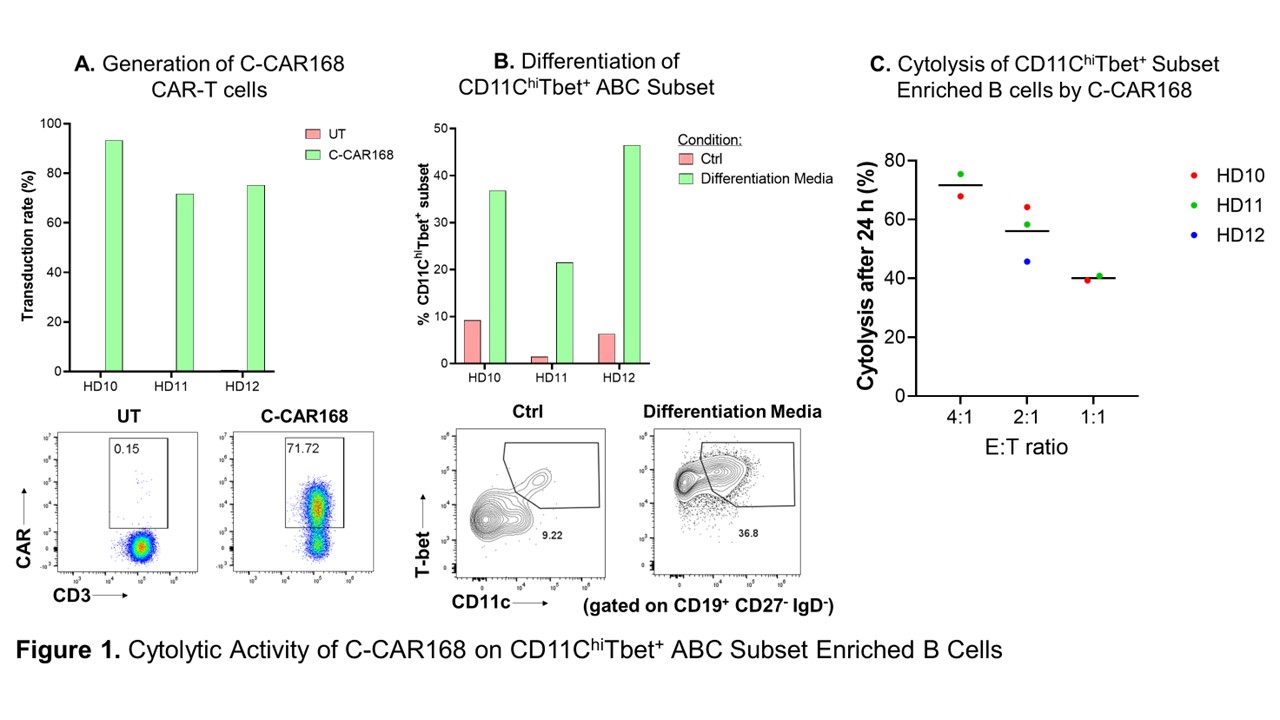
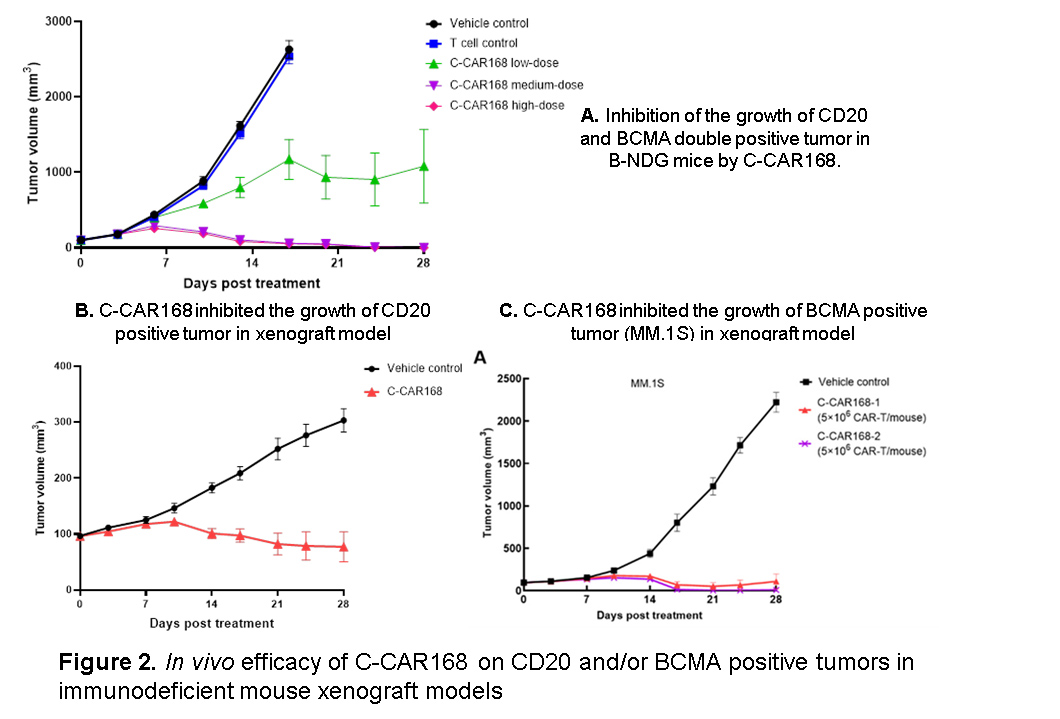
Results: Results of C-CAR168 cell function assays showed antigen specificity and reactivity of C-CAR168, and cell killing experiments demonstrated its specific cytotoxicity to CD20 and/or BCMA expressing target cells. Our results also demonstrated the activation and cell killing of C-CAR168 in the presence of in vitro generated age-associated B cells (ABC), a distinct B cell subset highly expanded in autoimmune diseases and associated with disease activity. In studies using immunodeficient mouse xenograft models, a single dose of C-CAR168 at 1×106, 5×106 and 10×106 CAR-T cells/mouse effectively inhibited the growth of CD20 and BCMA double positive target cells (K562-CD20-BCMA) in B-NDG mice. The in vivo efficacy of C-CAR168 on CD20 or BCMA single positive cells were also evaluated in A549-CD20 xenograft and MM.1S xenograft models, and C-CAR168 had anti-target cell activity at the dose level of 3×106 and 5×106 cells/mouse in both models. The results of membrane proteome array and healthy human tissue immunohistochemistry assays confirmed that C-CAR168 is specific to CD20 and BCMA proteins. In addition, using IL-2 independent growth and colony formation assays, we demonstrated that the transformation or tumorigenicity risk of C-CAR168 cells are minimal.
Conclusion: Based on the promising in vitro/in vivo efficacy results and safety data, C-CAR168 is being evaluated in clinical trials to treat patients with a variety of autoimmune diseases that are refractory to standard therapy.
To cite this abstract in AMA style:
Huang j, Yao X, Luo X, Lv X, Wei Y, Patrick M, Wang F, Hong Y, Yao Y. C-CAR168 as a Novel Anti-CD20/BCMA Bispecific Autologous CAR-T Therapy for the Treatment of Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/c-car168-as-a-novel-anti-cd20-bcma-bispecific-autologous-car-t-therapy-for-the-treatment-of-autoimmune-diseases/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/c-car168-as-a-novel-anti-cd20-bcma-bispecific-autologous-car-t-therapy-for-the-treatment-of-autoimmune-diseases/