Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Genetic variations in the OX40 ligand (OX40L) locus have been implicated in the susceptibility to systemic lupus erythematosus (SLE). Notably, the blockade of OX40L has demonstrated promising effects in mitigating renal damage and suppressing autoantibody production in NZB/W F1 mice, a commonly used model for SLE. Despite these encouraging findings, the precise mechanisms by which OX40L blockade exerts its delaying effect on the lupus phenotype remain elusive, warranting further investigation and exploration.
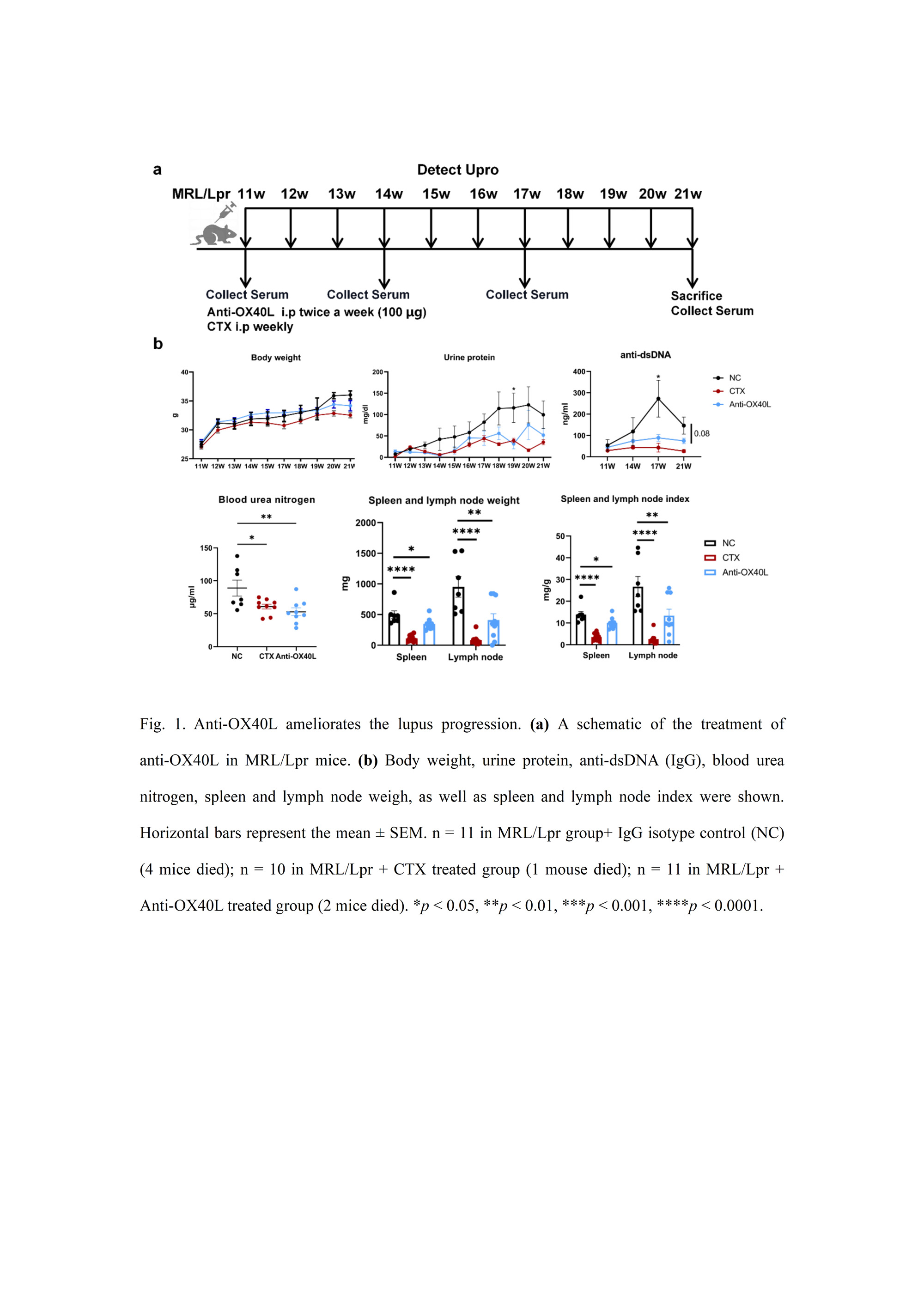
Methods: In the present study, we conducted an investigation to assess the effects of OX40L blockade using anti-OX40L in the MRL/lpr murine model of lupus, a well-established experimental system. The mice were categorized into three groups, each consisting of 9-11 individuals: IgG treatment, Cyclophosphamide (CTX) treatment, and anti-OX40L treatment. Following the respective treatments, the mice were sacrificed, and samples of serum, kidney, and spleen were collected for comprehensive outcome evaluation.
Subsequently, we explored the impact of anti-OX40L treatment on immunosuppression in 8-week-old C57BL/6J mice that were immunized with KLH. This was accomplished through the measurement of serum immunoglobulins (Igs) and flow cytometry analysis of splenocytes. Additionally, in vitro experimentation involving the treatment of anti-OX40L in CD4+ T cells and CD19+ B cells was conducted to elucidate the roles of OX40L in the pathogenesis of SLE.
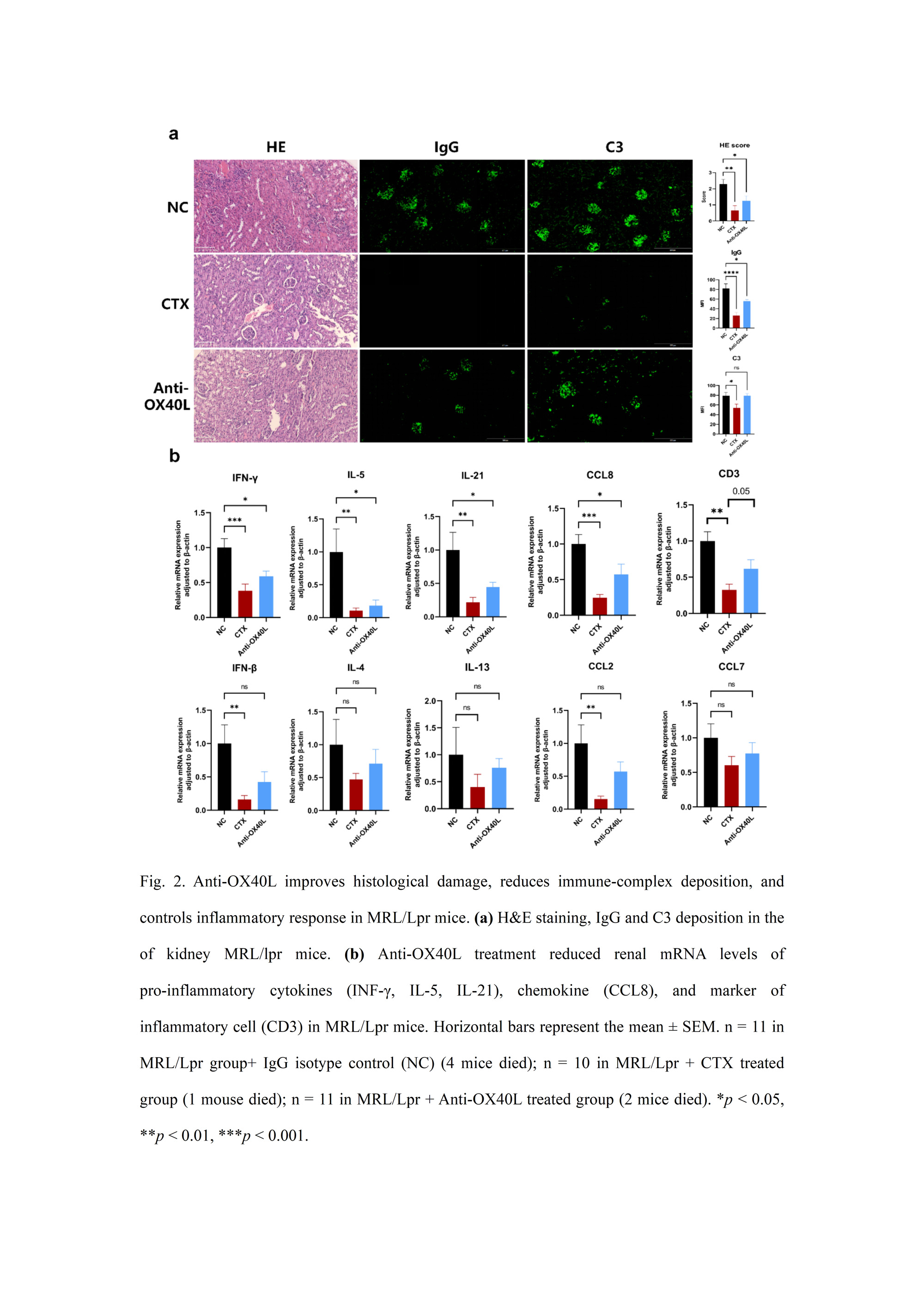
Results: In the current study, we have made significant observations regarding the impact of blocking OX40L in MRL/lpr mice, a murine model of lupus. Notably, treatment with anti-OX40L resulted in a remarkable delay in disease progression, as evidenced by reduced production of anti-dsDNA antibodies, decreased proteinuria, and diminished Ig deposition in the kidney. Additionally, we observed lower frequencies of Th1 and Tfh cells in the spleen of anti-OX40L-treated mice compared to the IgG-treated group.
Furthermore, our in vitro experiments revealed that anti-OX40L treatment promoted the up-regulation of polyclonal CD4+ T cell differentiation into regulatory T cells (Tregs). This finding suggests a potential mechanism through which anti-OX40L exerts its beneficial effects. In KLH-immunized mice, the administration of anti-OX40L resulted in decreased levels of immunoglobulins (Igs) and reduced numbers of plasmablast cells, further supporting the immunosuppressive effects of OX40L blockade.
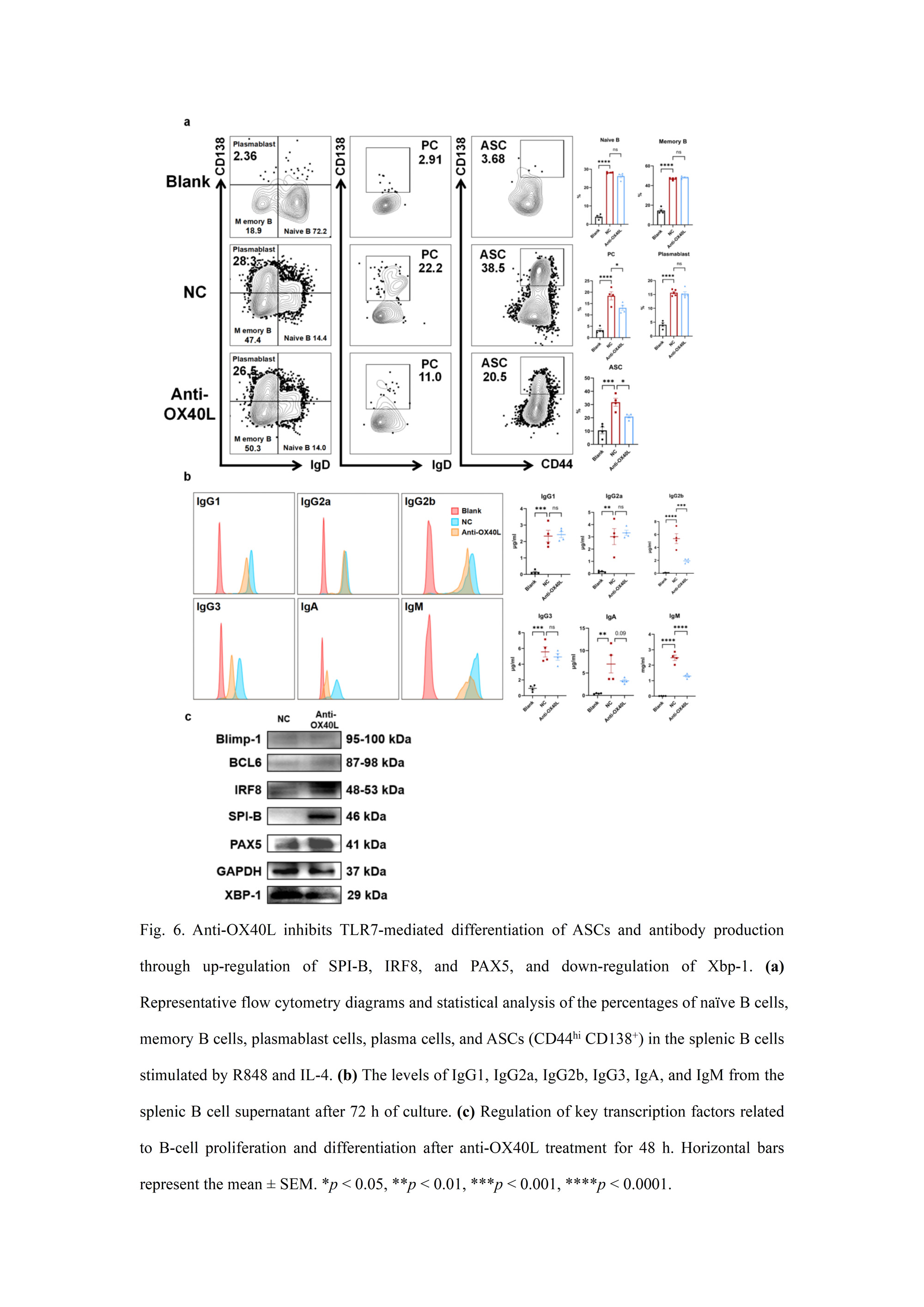
Interestingly, we also found that blocking OX40/OX40L signaling inhibited the TLR7-mediated differentiation of antibody-secreting cells (ASCs) and antibody production. This effect was accompanied by the up-regulation of SPI-B, IRF8, and PAX5, as well as the down-regulation of Xbp-1 in B cells, indicating the involvement of multiple regulatory factors in this process.
Conclusion: The results obtained from our study, along with the extensive evidence from previous investigations, underscore the potential of blocking OX40L as an effective strategy to mitigate disease progression and alleviate the pathological manifestations of SLE.
To cite this abstract in AMA style:
Zhao J, Li L, Feng X, Yin H, Lu Q. Blockade of OX40/OX40L Signaling Using anti-OX40L Ameliorates Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/blockade-of-ox40-ox40l-signaling-using-anti-ox40l-ameliorates-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/blockade-of-ox40-ox40l-signaling-using-anti-ox40l-ameliorates-systemic-lupus-erythematosus/