Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Available drugs to treat autoimmune diseases are indiscriminate, suppressing self-reactive and protective immune responses alike. This lack of therapeutic precision results in infection and excess mortality. CD19-CAR-T cells have demonstrated remarkable potency in the treatment of severe autoimmune diseases, but are resource intensive, broadly immunosuppressive, and require cytotoxic therapy (“conditioning”). In contrast, precision therapies that can eliminate autoreactive B cells with selectivity have the potential to treat, control, and prevent autoimmune diseases without impairing protective immunity. Here, we aimed to develop an “off-the-shelf” precision immunotherapy, bispecific autoantigen-T cell engaging (BaiTE) antibodies, for the treatment of APS. We hypothesized that BaiTEs can redirect T cells to selectively eliminate autoreactive B cells against beta-2 glycoprotein I (B2GPI), a key driver in APS.
Methods: Ramos B cells were CRISPR-Cas9-edited to replace their B-cell receptors (BCRs) with anti-B2GPI BCRs cloned from APS patients. BaiTEs, fusion proteins comprising truncations of the APS autoantigen B2GPI (DI, DI-II, DI-III, DI-IV, DI-V) and anti-CD3 single-chain variable fragments (scFvs), were cloned and expressed in mammalian cells. Binding of BaiTEs to anti-B2GPI B cells, T cells, and CD3δ/ε was quantified. B2GPI was mutated to eliminate off-target binding. BaiTE affinities to immobilized BCRs were determined by surface plasmon resonance (SPR). The potency and specificity of BaiTEs against anti-B2GPI Ramos B cells were interrogated in co-culture. Cytotoxicity was quantified by flow cytometry, live-cell imaging, and cytokine assays. Autoantibody depletion was measured by ELISA.
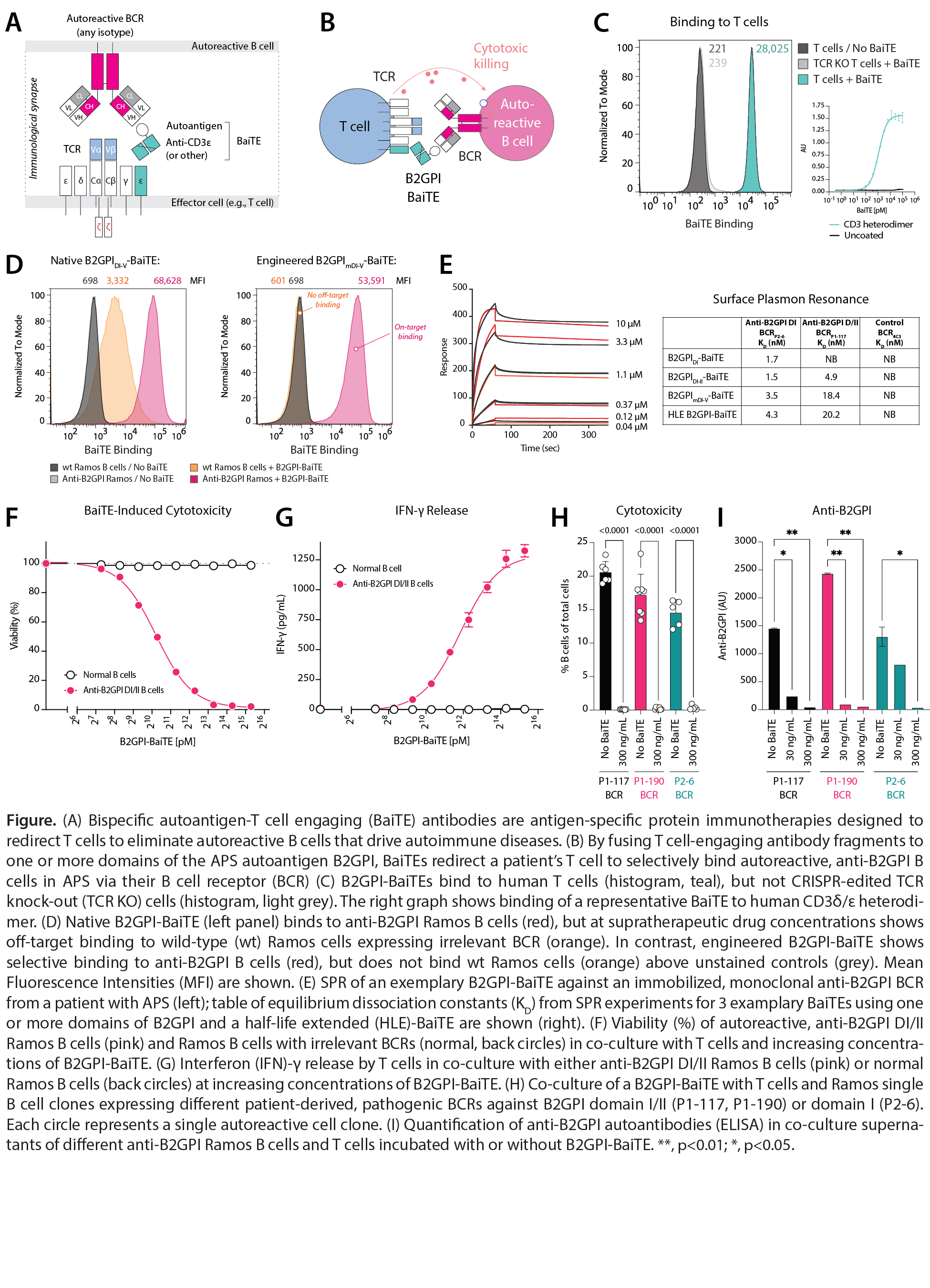
Results: We engineered BaiTEs to selectively bind anti-B2GPI BCRs in APS, thereby redirecting T cells to kill autoreactive B cells (A-B). Binding of BaiTEs to T cells was dependent on TCR expression/CD3ε (C). Optimized B2GPI-BaiTEs specifically bound anti-B2GPI Ramos B cells (D). Binding affinities (KD) to immobilized anti-B2GPI BCRs by SPR were 1.5-20 nM, ~1000-fold higher than reported for anti-B2GPI antibodies (E). BaiTEs selectively eliminated IgG, IgA, and IgM anti-B2GPI B cells in a dose-dependent manner but spared normal B cells (F). Cytotoxicity induced by BaiTEs did not require exogenous cytokines and was observed at low BCR densities. Interferon-γ release was only observed with anti-B2GPI B cells (G). Treatment with BaiTE eliminated patient-derived anti-B2GPI B cell clones (H) and abrogated autoantibody production (I).
Conclusion: We developed an off-the-shelf precision immunotherapy, BaiTE, for the antigen-specific depletion of autoreactive B cells in patients with APS. BaiTEs selectively eliminate anti-B2GPI B cells—pathogenic drivers of thrombosis and fetal loss—in a dose-dependent manner, whilst sparing normal immune cells. BaiTEs overcome fundamental limitations of current drugs by combining the exquisite potency of T cell-engaging antibodies with therapeutic precision. These antigen-specific immunotherapies are disease-specific, maximally scalable, and promise a future of treating rheumatic diseases without increasing the risk of infection.
To cite this abstract in AMA style:
Xia Y, Liu J, Pearlman A, Mog B, Shaw E, Kaeo K, Gliech C, Moritz B, Awosika T, DiNapoli S, Glavaris S, Ge J, Nichakawade T, Marcou N, Paul S, Pardoll D, Bettegowda C, Goldman D, Petri M, Rosen A, Kinzler K, Zhou S, Vogelstein B, Konig M. Bispecific Autoantigen-T Cell Engagers (BaiTE) to Selectively Target Autoreactive B Cells in Antiphospholipid Syndrome [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/bispecific-autoantigen-t-cell-engagers-baite-to-selectively-target-autoreactive-b-cells-in-antiphospholipid-syndrome/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bispecific-autoantigen-t-cell-engagers-baite-to-selectively-target-autoreactive-b-cells-in-antiphospholipid-syndrome/