Session Information
Date: Sunday, November 13, 2022
Title: Plenary II
Session Type: Plenary Session
Session Time: 11:30AM-1:00PM
Background/Purpose: In routine care, biosimilar-to-biosimilar infliximab switching may occur to save costs (=non-medical switching). Previous studies have investigated the efficacy and safety of switches from originator infliximab to a corresponding biosimilar in patients with inflammatory rheumatic diseases (1). However, the outcomes after switching from one infliximab biosimilar to a second infliximab biosimilar remain scarcely investigated. Denmark has recently conducted a nationwide mandatory infliximab biosimilar-to-biosimilar switch. In this study, we aimed to investigate the effectiveness of switching infliximab biosimilars CT-P13-to-GP1111 among patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA) and axial spondyloarthritis (AxSpA), including patients who had previously switched from originator (originator-experienced) to CT-P13 as well as patients who were originator-naïve.
Methods: Observational cohort study based on DANBIO registry linked with national patient registries (to identify prior comorbidities). Patients with RA, PsA or AxSpA who performed a biosimilar-to-biosimilar switch from CT-P13 to GP1111 between April 1st 2019 and February 1st 2020 were included. Patient were divided into two groups: originator-naïve and originator-experienced. Main outcomes in the two groups were one-year GP1111 treatment retention (Kaplan Meier “drug survival curves”) and changes in disease activity 4 months before versus 4 months after switch in individual patients. Also, we identified clinical factors at baseline (=at time of switch) associated with withdrawal (multivariable Cox-regression analyses with Hazard Ratios (HR) including originator treatment history).
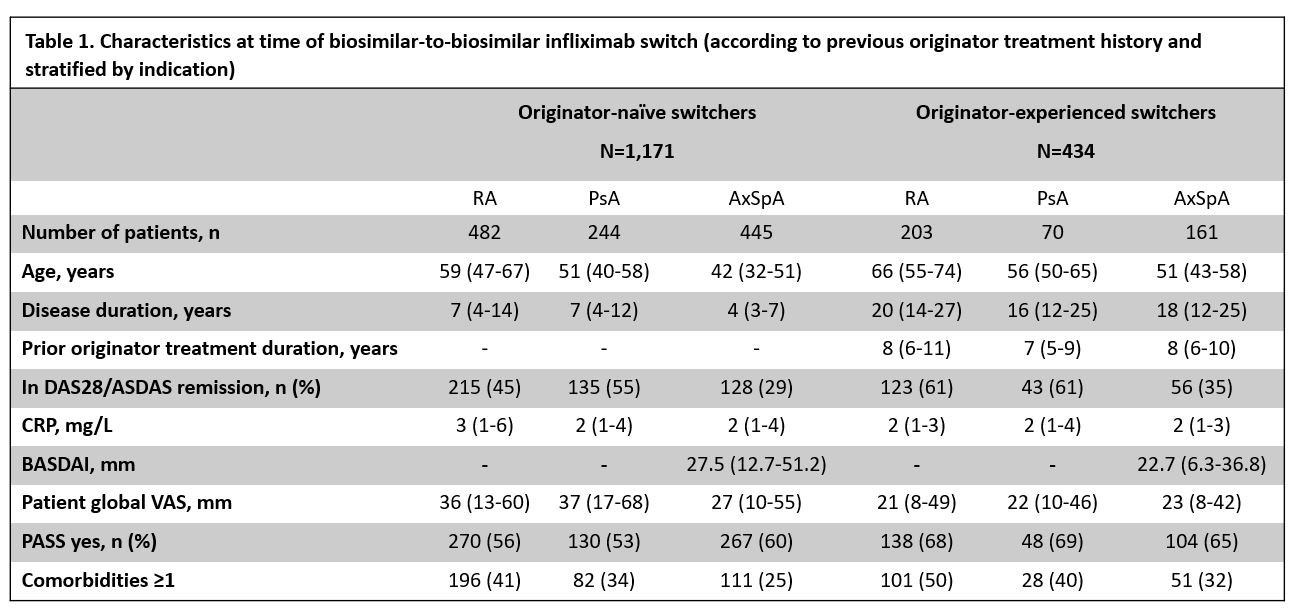
Results: Of 1,605 patients (685 RA/314 PsA/606 AxSpA, median disease duration 9 years, 37% in CDAI/ASDAS remission), 1,171 were originator-naïve (Table 1).
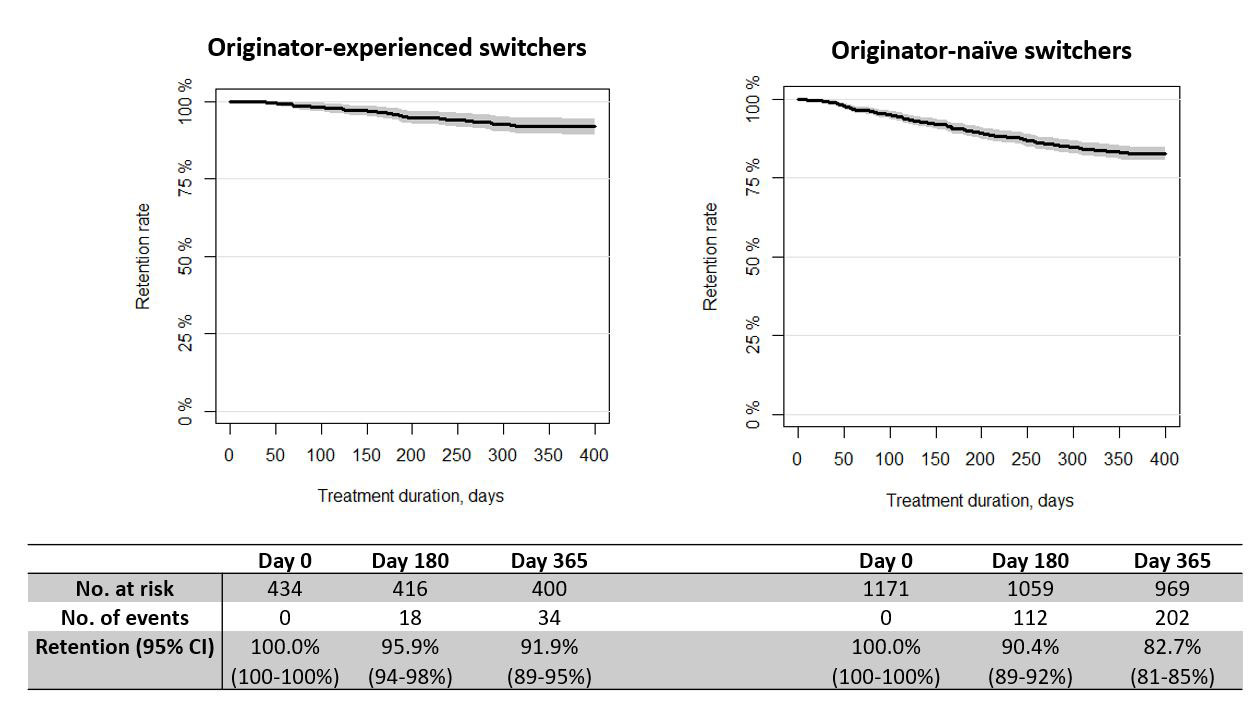
One-year retention rates of GP111 were 83% (95% confidence interval (CI): 81-85%) and 92% (90-95%) for the originator-naïve and originator-experienced, respectively (Figure 1). Changes in disease activity 4 months pre- and post-switch were close to zero for all disease activity measures (e.g. DAS28, ASDAS, VAS pain, not shown).
Factors associated with higher GP1111 retention rates were being originator-experienced compared to originator-naïve in RA (HR=0.4 (95%CI:0.2-0.7)) and PsA (HR=0.2 (0.1-0.8)), but not in AxSpA: HR=0.6 (0.3-1.2), and lower disease activity at baseline (Table 2).
Conclusion: In this real-world observational study of >1,500 patients with inflammatory arthritis, one-year retention following a mandatory nationwide infliximab biosimilar-to-biosimilar switch was high. Retention was higher in originator-experienced and in patients with low disease activity, suggesting outcomes to be affected by patient- rather than drug-related factors.
To cite this abstract in AMA style:
Nabi H, Hendricks O, Jensen D, Loft A, Pedersen J, Just S, Danebod K, Munk H, Kristensen S, Manilo N, Colic A, Linauskas A, Thygesen P, Christensen L, Kalisz M, Lomborg N, Chrysidis S, Raun J, Andersen M, Mehnert F, Krogh N, Hetland M, Glintborg B. Biosimilar-to-Biosimilar Switching in Routine Care – Results on >1,600 Patients with Inflammatory Arthritis in the DANBIO Registry [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/biosimilar-to-biosimilar-switching-in-routine-care-results-on-1600-patients-with-inflammatory-arthritis-in-the-danbio-registry/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biosimilar-to-biosimilar-switching-in-routine-care-results-on-1600-patients-with-inflammatory-arthritis-in-the-danbio-registry/