Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Early initiation of treatment with the recombinant Interleukin 1 receptor antagonist (IL-1Ra), anakinra, in Still’s disease (SD), specifically systemic Juvenile Idiopathic Arthritis, patients is safe and effective1,2. However, anakinra is also relatively expensive, not without side effects, and comprises a high burden due to the daily injections. Therefore, we have previously proposed and tested a taper-and-stop strategy for anakinra treatment in SD where patients with clinically inactive disease (CID) at time point 3 months after the start of anakinra were tapered and stopped. Around 50% of patients achieved remission without medication at time point 1 year after diagnosis1,2. Retrospectively, we found that the patients that relapsed after taper and stop, showed significantly higher levels of IL-18 prior to the start of tapering than the patients with a successful taper and stop attempt, suggesting that IL-18 levels might be used as a biomarker to taper and stop treatment. The current national prospective, multi-center, intervention study ESTIS (Early Stop of Targeted Treatment in Systemic JIA) aims to develop a safe and personalized ‘taper and stop strategy’ for SD patients with a complete response to first-line use of rIL-1RA.
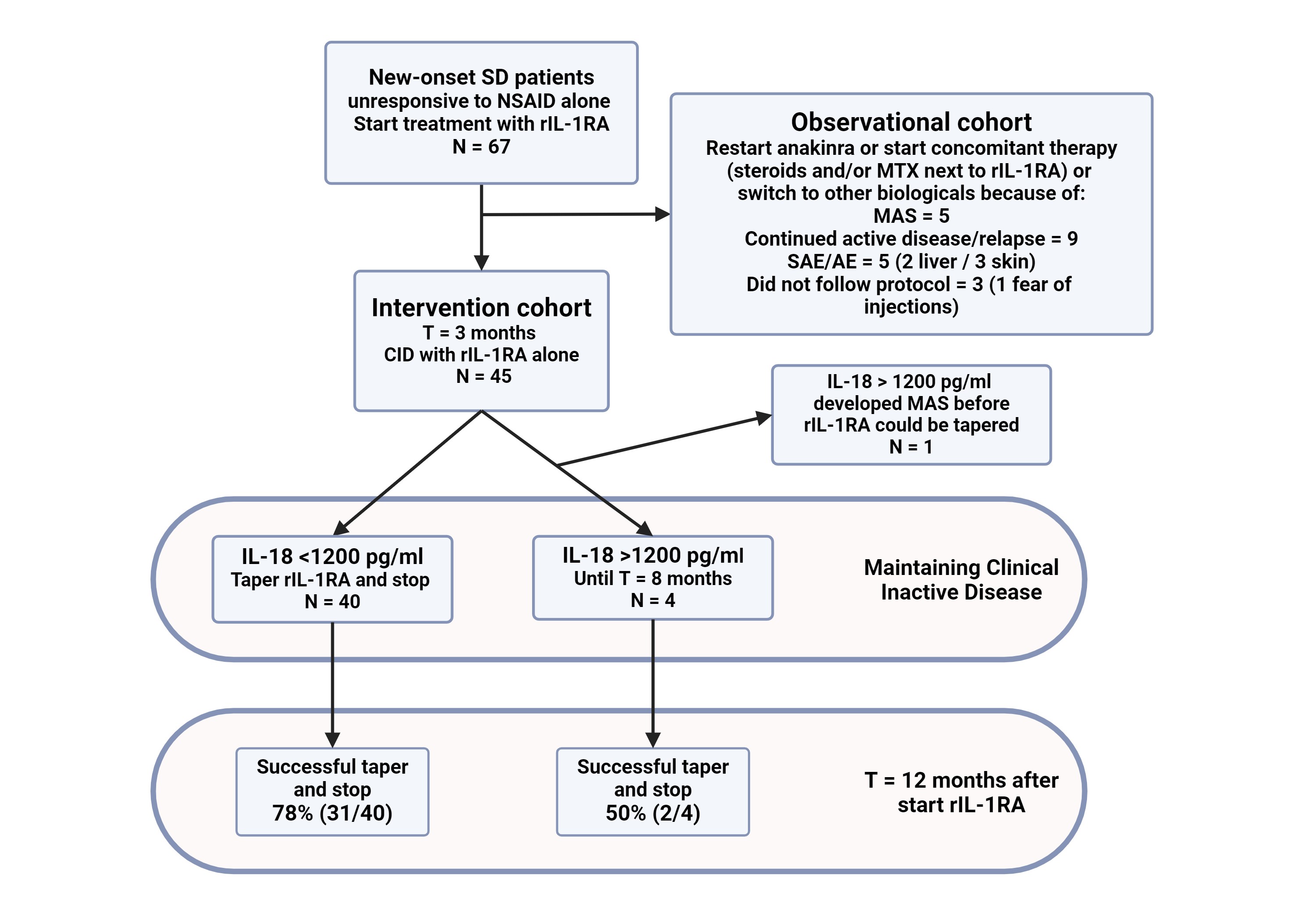
Methods: The study consisted of an open-label lead-in phase, in which patients with new-onset, biological and steroid naïve SD, from 6 centers in the Netherlands were included. SD diagnosis was made according to the PRINTO criteria3. Serum levels of IL-18 were measured by Luminex technology1. All patients received anakinra as first-line therapy with frequent follow-up and sampling. Only patients with a good initial clinical response and achievement of CID at T=3 months after start on rIL-1RA mono-therapy were allowed to enter the intervention study: these patients were assessed every month for both clinical response and IL-18 levels. Patients with IL-18 < 1200 pg/mL were switched to an alternate day regimen and the rIL-1RA was subsequently stopped 1 month later. If the IL-18 remained above the threshold of 1200 pg/mL, rIL-1RA was continued in a daily dose. Patients with CID at t=9 months after start of treatment were tapered and stopped regardless of IL-18 levels. All patients were followed up to 2 years after start of treatment.
Results: In total, 67 patients, were enrolled in the study of which 44 showed CID on rIL-1RA monotherapy at t=3 months, allowing entry in the intervention phase of the trial (Figure 1). At baseline, there was no significant difference in the clinical and laboratory characteristics between the later intervention (CID) and the non-intervention (more refractory course up to 3 months) group except for the percentage of females, 43% and 79% respectively (p=0.004). In the intervention group, 75% (33/44) of SD patients were successfully tapered and stopped, with a sustained clinical response at t=12 months. This is significantly (p=0.043) better than the percentage of successful taper and stop in our historic cohort (47%, 7/15)1.
Conclusion: Our prospective, multicenter intervention trial shows that IL-18 with a cut-of value of 1200 pg/mL is helpful in successful tapering and stop of rIL-1RA in well-responding SD patients.
1. PMID: 24757154
2. PMID: 30848528
3. PMID: 35105709
CID = Still’s disease, encompassing systemic Juvenile Idiopathic Arthitis and Adult onset Still’s Disease
rIL_1RA = Recombinant Interleukin_1 Receptor Antagonist (anakinra)
MAS = Macrophage Activation Syndrome
(S)AE = (Serious) Adverse Event
CID = Clinical Inactive Disease
To cite this abstract in AMA style:
Erkens R, Den Engelsman G, De Roock S, Hamann D, Schonenberg-Meinema D, van den Berg M, Gruppen M, Armbrust W, Legger E, Kamphuis S, Verkaaik M, Schatorjé E, Hoppenreijs E, Vogl T, Roth J, Hissink Muller P, Swart J, Jansen M, van Loosdregt J, Vastert S. Biomarker-guided Treatment-and-stop-strategy for Recombinant IL-1Receptor Antagonist (Anakinra) in Patients with Systemic Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/biomarker-guided-treatment-and-stop-strategy-for-recombinant-il-1receptor-antagonist-anakinra-in-patients-with-systemic-juvenile-idiopathic-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biomarker-guided-treatment-and-stop-strategy-for-recombinant-il-1receptor-antagonist-anakinra-in-patients-with-systemic-juvenile-idiopathic-arthritis/