Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The idiopathic inflammatory myopathies represent a group of autoimmune diseases that target muscle as well as extra-muscular organs, leading to significant morbidity and mortality. Among the most common specific autoantibodies associated with these disorders is Anti-histidyl-tRNA synthetase (HRS; Jo-1), which defines a subgroup of patients with clinical features. In order to further advance targeted therapies, we have modified a previously established model of HRS-induced myositis to highlight the potential role of NF-kB activation in early stages of disease.
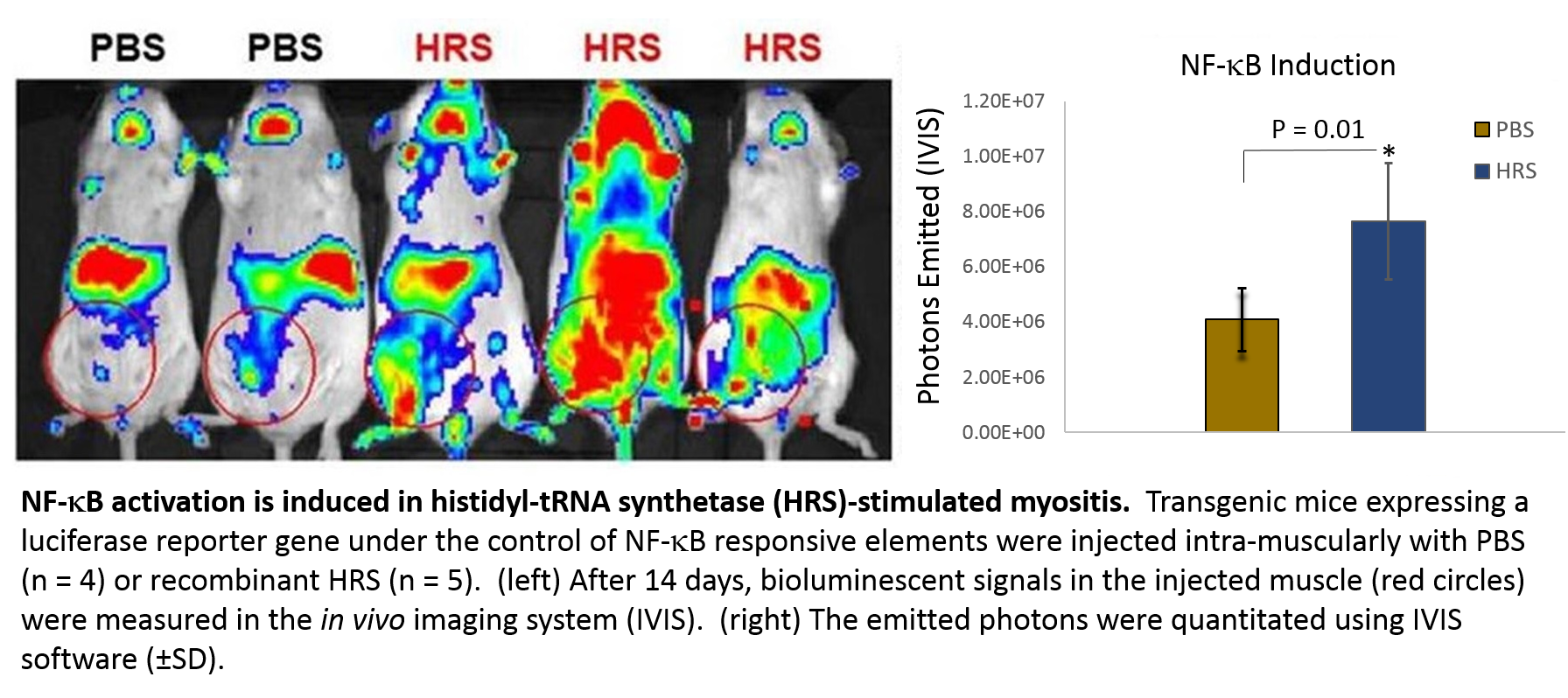
Methods: BALB/C-Tg(NFkB-RE-luc)-Xen mice, which contain a firefly luciferase cDNA reporter gene under the regulation of 3 kB responsive binding sites, were injected intra-muscularly with 50 ml of recombinant murine HRS (5 mg/ml) affinity purified following amplification in a bacterial expression system. Inflammation was determined by measuring whole-body bioluminescent signals using the Xenogen in vivo imaging system (IVIS 200). The emitted photons from injected muscle were quantitated for each mouse at time zero and at 2 and 4 weeks. At 5 weeks post-HRS injection, mice were sacrificed; sections of injected as well as non-injected muscle tissue were then paraffin embedded and stained by H&E for histological analysis.
Results: NFkB-RE-luc mice inoculated with recombinant HRS developed a robust inflammatory response at the 2 week time point. This statistically significant inflammatory response measured by IVIS photon quantification was most pronounced at the site of injection, but did extend beyond this area in some mice (see figure). NF-kB activation subsided after 4 weeks, with residual bioluminescent signals approaching those induced by injection with PBS alone. Despite this apparent reduction in NF-kB activity, however, histologic analysis of HRS-injected muscle tissue revealed significant endomysial inflammatory infiltrates at these later time points.
Conclusion: This novel application of NF-kB-regulated luciferase mice establishes a system that may facilitate therapeutic drug development for myositis through longitudinal analysis of candidate NF-kB inhibitors in different strains expressing the NF-kB-luciferase transgene. However, because our results suggest that NF-kB-mediated signaling pathways primarily impact early stages of disease in this model system, alternative therapeutic targets must be sought for more temporally advanced disease; thus underscoring the need for phase-specific treatment in idiopathic inflammatory myopathy.
Disclosure:
N. A. Young,
None;
L. C. Wu,
None;
M. Bruss,
None;
W. N. Jarjour,
None;
D. P. Ascherman,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bioluminescent-imaging-of-histidyl-transfer-rna-synthetase-induced-myositis-reveals-early-phase-involvement-of-nf-kb-mediated-inflammation/