Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Inactive disease is the goal for youth with spondyloarthritis (SpA). Many patients are interested in stopping medications after inactive disease is achieved. The risk of flare in youth with SpA after de-escalating therapy with a tumor necrosis factor inhibitor (TNFi) is unknown. The BACK-OFF JSpA Study aims to evaluate the risk of disease flare and patients’ lived experiences using different TNFi de-escalation strategies.
Methods: BACK-OFF JSpA is a randomized pragmatic multicenter trial of youth with SpA who have sustained inactive disease while being treated with a TNFi medication. Subjects are randomly assigned 1:1:1 to: 1) continue standard TNFi dose, 2) increase time between TNFi doses, or 3) stop TNFi. Randomization is stratified by axial disease and polyarticular course. Study visits occur every 3 months for up to one year, or flare, and consist of a physical exam and surveys that ask about patient’s lived experiences. Ancillary studies are evaluating the development of anti-drug antibodies and the association of serologic (all subjects eligible) and imaging (subjects with MRI-defined axial disease eligible) biomarkers with flare risk. Patient and parent stakeholders helped to design and conduct this study and created all recruitment materials.
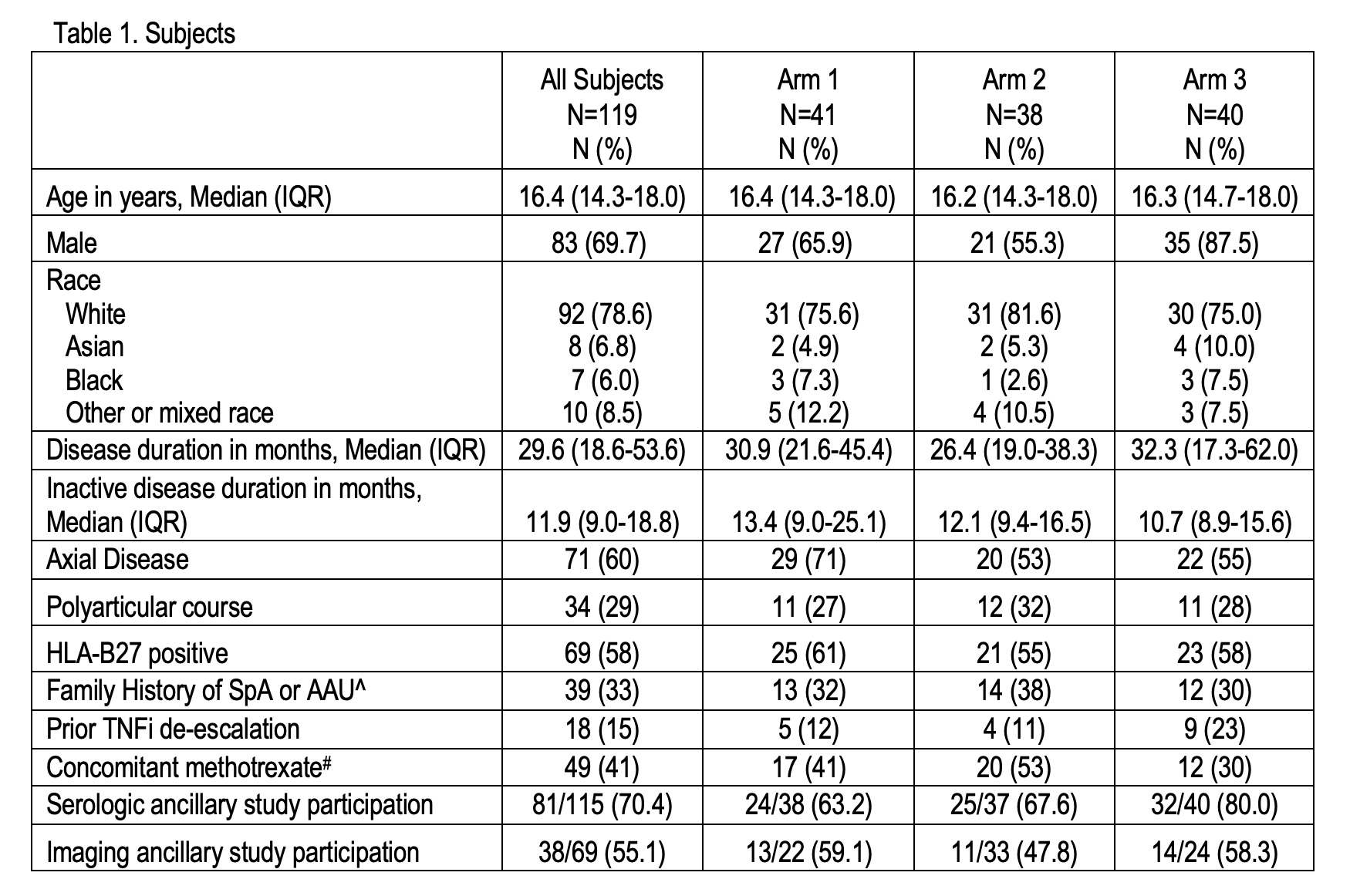
Results: 119 youth are enrolled across 29 sites, which is 60% of the total study goal. Table 1 lists subject characteristics. 69% are male and the median age is 16.4 years. 58% are HLA-B27 positive. 60% and 29% of subjects have a history of axial or polyarticular disease, respectively. At time of enrollment, the median disease duration was 29.6 months and median time of sustained inactive disease was 11.9 months (IQR 9.0-18.8). At enrollment 79%, 13%, 3%, and 5% of subjects were on standard doses of adalimumab, etanercept, golimumab, or infliximab, respectively. 38%, 3%, and 0% were also on methotrexate, sulfasalazine, or leflunomide, respectively. 15% subjects previously tried de-escalating TNFi therapy. 28 of 29 sites enrolled ≥ 1 subject and 15 sites enrolled ≥ 4 subjects (Figure 1). 47% of subjects enrolled at the 2nd or 3rd recruitment attempt. 70% (N=81/115) and 55% (N= 38/69) of subjects participated in the serologic and/or imaging ancillary studies. Of the enrolled patients 66% acknowledged viewing recruitment materials and 64% rated their impact on decision to enroll as positive (Table 2). The top reasons for declining the study include: “Doesn’t want to change treatment” (59%), “Not willing to be randomized” (26%), and “Did not have sufficient time to enroll” (5%). So far, 65 subjects completed the active intervention period.
Conclusion: Enrollment in the BACK-OFF JSpA trial is ongoing and over halfway to the goal. Enrollment for the main trial and ancillary studies are robust across sites. Stakeholder-partner designed recruitment materials are influential in a family’s decision to enroll. Most families are willing to be reapproached about the study at more than 1 visit.
To cite this abstract in AMA style:
Sears C, Muir C, Brandon T, Ferguson P, Correll C, Rosenkranz M, Baszis K, Lee T, Oberle E, Stoll M, Cook K, Muscal E, Srinivasalu H, Lovell D, Prahalad S, Cidon M, Mulvihill E, Klein-Gitelman M, Kingsbury D, Cooper J, Rosenwasser N, Treemarcki E, Chang J, Tarvin S, Walters H, Shishov M, Buckley L, Toth M, Cooper A, Xiao R, Neu E, Kohlheim M, Leal J, Archie K, Holland E, Holland M, Hameed A, Khan A, Murphy L, Murphy S, Neu J, Richmond R, Suplee D, Suplee T, Wiley D, Weiss P. Biologic Abatement and Capturing Kids Outcomes and Flare Frequency in Juvenile Spondyloarthritis: Baseline Characteristics and Enrollment [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/biologic-abatement-and-capturing-kids-outcomes-and-flare-frequency-in-juvenile-spondyloarthritis-baseline-characteristics-and-enrollment/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biologic-abatement-and-capturing-kids-outcomes-and-flare-frequency-in-juvenile-spondyloarthritis-baseline-characteristics-and-enrollment/