Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: We have recently shown that anti-phospholipid (APL) assays of varying methodology demonstrate comparable analytical performance despite variable levels of agreement among individual analytes, identifying binding patterns among ELISA and non-ELISA aPL assays for each analyte. This follow-up study evaluates binding characteristics of anticardiolipin (aCL) and anti-β2-glycoprotein I (aβ2GPI) antibodies underlying these patterns that could potentially contribute to APL inter-assay variability.
Methods: Aliquoted sera from 100 individuals (57 female); 56 patients with clinical disease (n=12 APS, n=18 APS/SLE, n=26 diverse autoimmune diseases including 7 with SLE), and 44 healthy controls were evaluated in 9 clinical laboratories. All specimens were tested for aCL and aβ₂GPI IgG, IgM, IgA antibodies in 3 non-ELISA (A1-A3) and 6 ELISA (K1-K6) kits using four different methods [ELISA, chemiluminescence(CIA), fluoro-enzyme (FEIA), and multiplexed bead (MPlex)] from 7 manufacturers. An exploratory principal component analysis (PCA) evaluated binding patterns and agglomerative hierarchical clustering (AHC) based on Ward’s criterion defined patient clusters among assays of each APL analyte. Avidity in aCL and aβ2GPI assays was evaluated according to a published protocol using modified K4 kits, reported as percentage binding retention when sample buffer solute concentration was increased 2 and 5 times normal [Artenjak et al 2015]. Cofactor-dependent aCL binding was evaluated using a modified K4 aCL assay free of β2GPI cofactor in all components. The effect of increasing β2GPI (12.5 to 50ug/ml) in sample buffer on the binding activity OD of each sample was determined [Papalardo et al 2017].
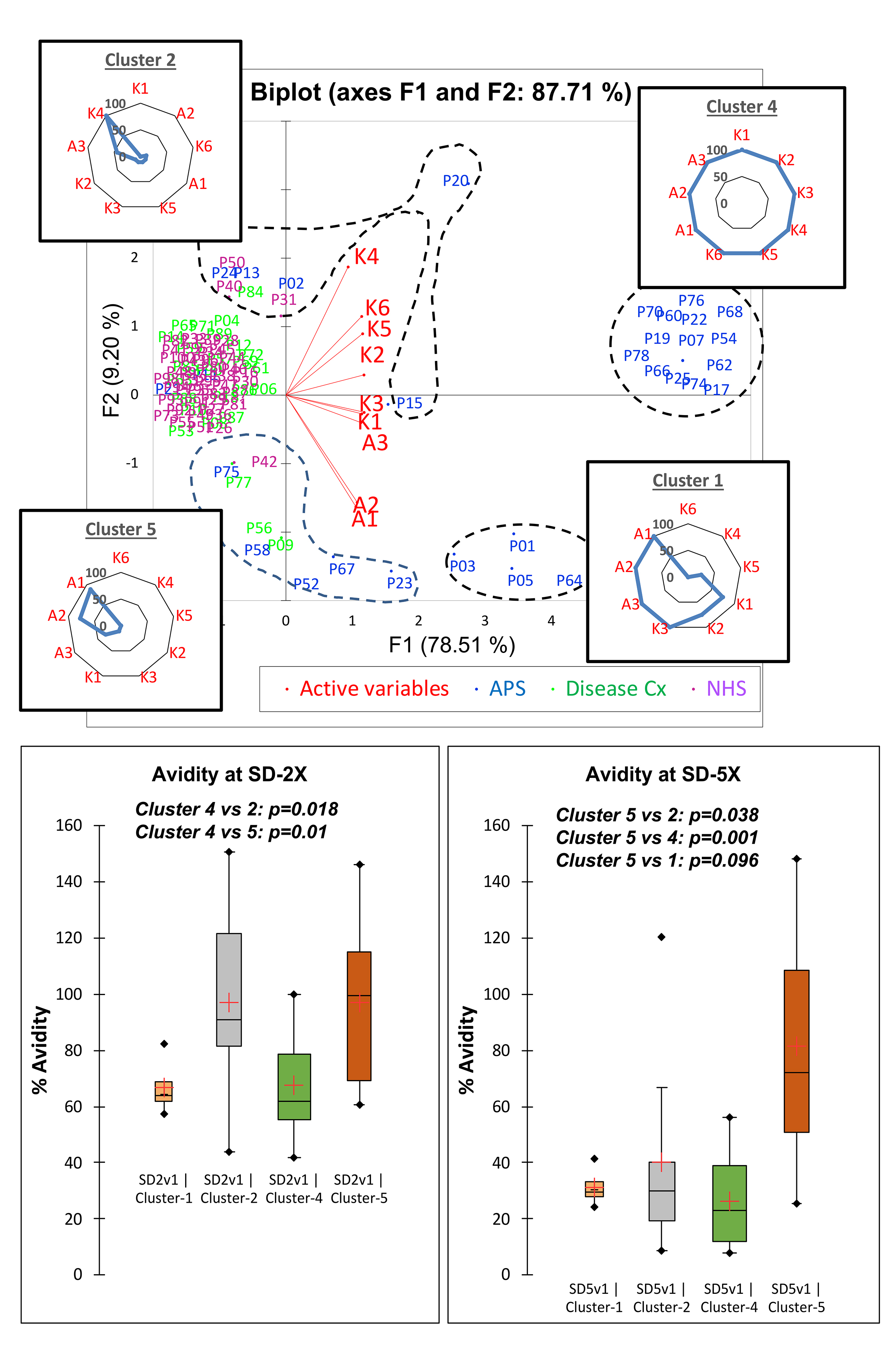
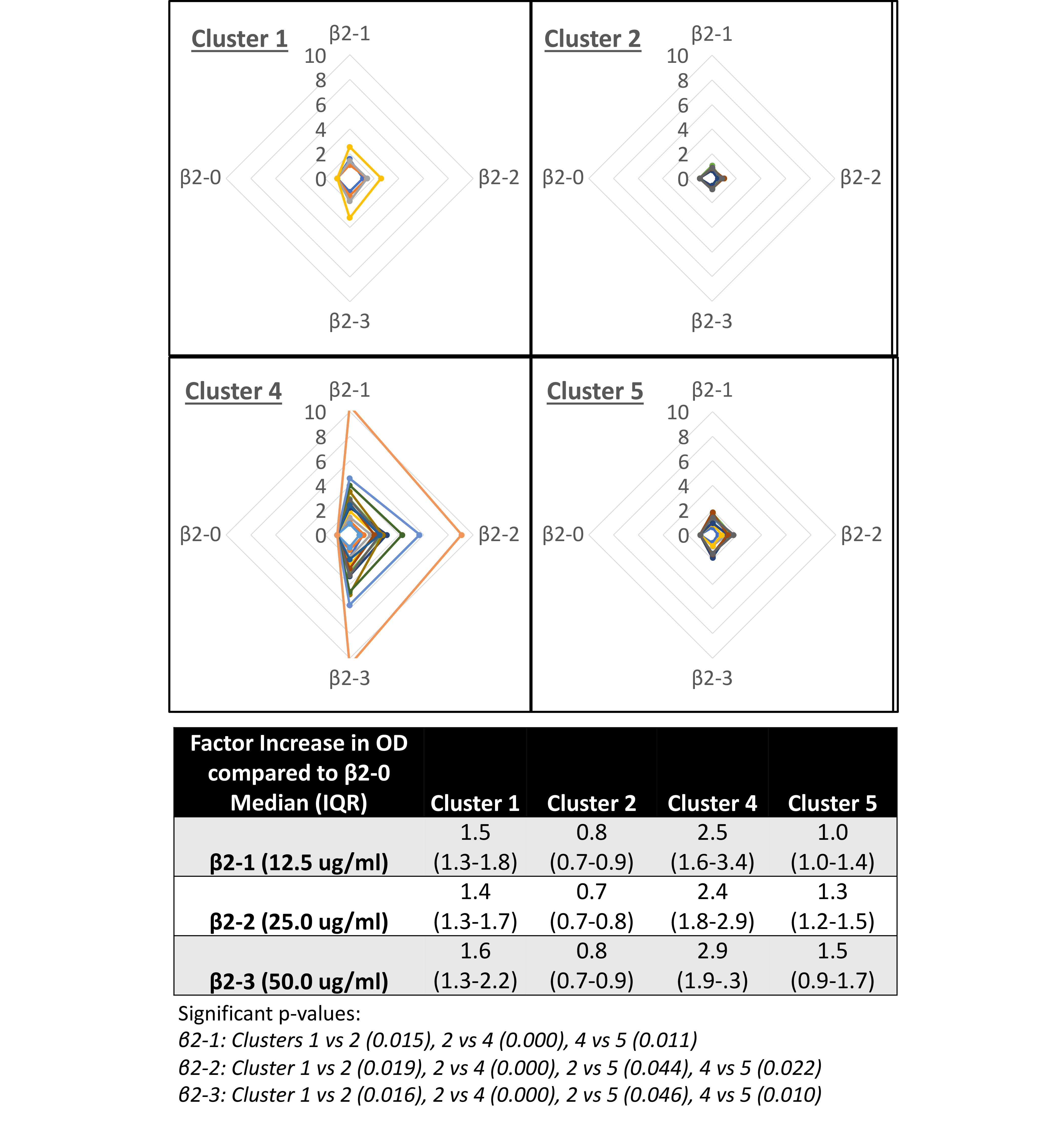
Results: In PCA biplots, non-ELISA (A1-A3) and ELISA (K1-K6) assays tend to cluster together, with A3 (FEIA) often falling between the two clusters. AHC analysis identified distinct patient clusters within each aPL analyte, often identifying clusters with broad reactivity (eg. clusters 1 and 4 – IgG aCL) across most or all assays and clusters with very narrow binding for 1 or 2 assays (eg. Clusters 2 and 5 – IgG aCL) (Figure 1). For IgG aCL, clusters 1 and 4 were all APS patients, while 2 and 5 both had 56% APS. At 2x buffer concentration, across all aCL and aβ2GPI analytes, binding retention ranged from 61 to 80% for broad reactivity clusters compared to 85 -107% for narrow reactivity clusters with a similar pattern at 5x buffer concentration (IgG aCL – Figure 1). In the cofactor-dependent IgG aCL assay, median OD change (1.4 to 2.9 times increase) in broad reactivity clusters was higher than that (0.7 to 1.5) seen in narrow reactivity clusters (p< 0.001) (Figure 2), with a similar pattern in IgM and IgA aCL.
Conclusion: In this cohort including APS patients, aPL-positive samples displaying the broadest binding reactivity across kits for each analyte had the lowest avidity and the most significant cofactor-dependent increase in aCL binding. The variation of these binding characteristics of aPL antibodies in patient samples could partially explain inter-assay variation in aPL assays. Further analysis of epitope specific binding and the cross-reactivity of aCL and aβ2GPI will help to more clearly characterize the noted binding patterns.
To cite this abstract in AMA style:
Willis R, Murthy V, Roye-Green K, Thees A, Choi M, Dier K, Fritzler M, Fink S, Nandakumar V, Snyder M, Tebo A. Binding Characteristics of Patient Clusters Among Anticardiolipin and Anti-β2-Glycoprotein I ELISA and Non-ELISA Assays: A Survey by the Association of Medical Laboratory Immunologists [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/binding-characteristics-of-patient-clusters-among-anticardiolipin-and-anti-%ce%b22-glycoprotein-i-elisa-and-non-elisa-assays-a-survey-by-the-association-of-medical-laboratory-immunologists/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/binding-characteristics-of-patient-clusters-among-anticardiolipin-and-anti-%ce%b22-glycoprotein-i-elisa-and-non-elisa-assays-a-survey-by-the-association-of-medical-laboratory-immunologists/