Session Information
Date: Saturday, November 12, 2022
Title: Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Bimekizumab (BKZ) is a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A. BKZ improved signs and symptoms and reduced disease activity up to Week (Wk) 24 in patients (pts) with active non-radiographic axSpA (nr-axSpA) and active ankylosing spondylitis (AS; i.e. radiographic axSpA) in the phase 3 studies BE MOBILE 1 (NCT03928704) and BE MOBILE 2 (NCT03928743), respectively; all primary and ranked secondary endpoints at Wk 16 were met, including change from baseline (CfB) in nocturnal spinal pain.1,2 Here, we evaluate the impact of BKZ in pts with nr-axSpA and AS on major contributors to disease burden (spinal pain, stiffness and fatigue)3 to Wk 24 in both studies.
Methods: BE MOBILE 1 and 2 were conducted in parallel and had similar designs, with a 16-wk double-blind period followed by a 36-wk maintenance period. Pts were randomized to BKZ 160 mg Q4W or placebo (PBO); all pts received BKZ 160 mg Q4W from Wk 16 onward.1,2 We report proportion of pts achieving selected thresholds at Wk 16 for low pain (total and nocturnal spinal pain score: ≤0/1/2/3/4) and improvement in fatigue (Functional Assessment of Chronic Illness [FACIT]-Fatigue score: ≥4-point increase from baseline [BL]) using non-responder imputation. Mean CfB to Wk 24 in total spinal pain, nocturnal spinal pain, BASDAI morning stiffness (mean of BASDAI questions 5 and 6), and FACIT-Fatigue scores are reported using multiple imputation.
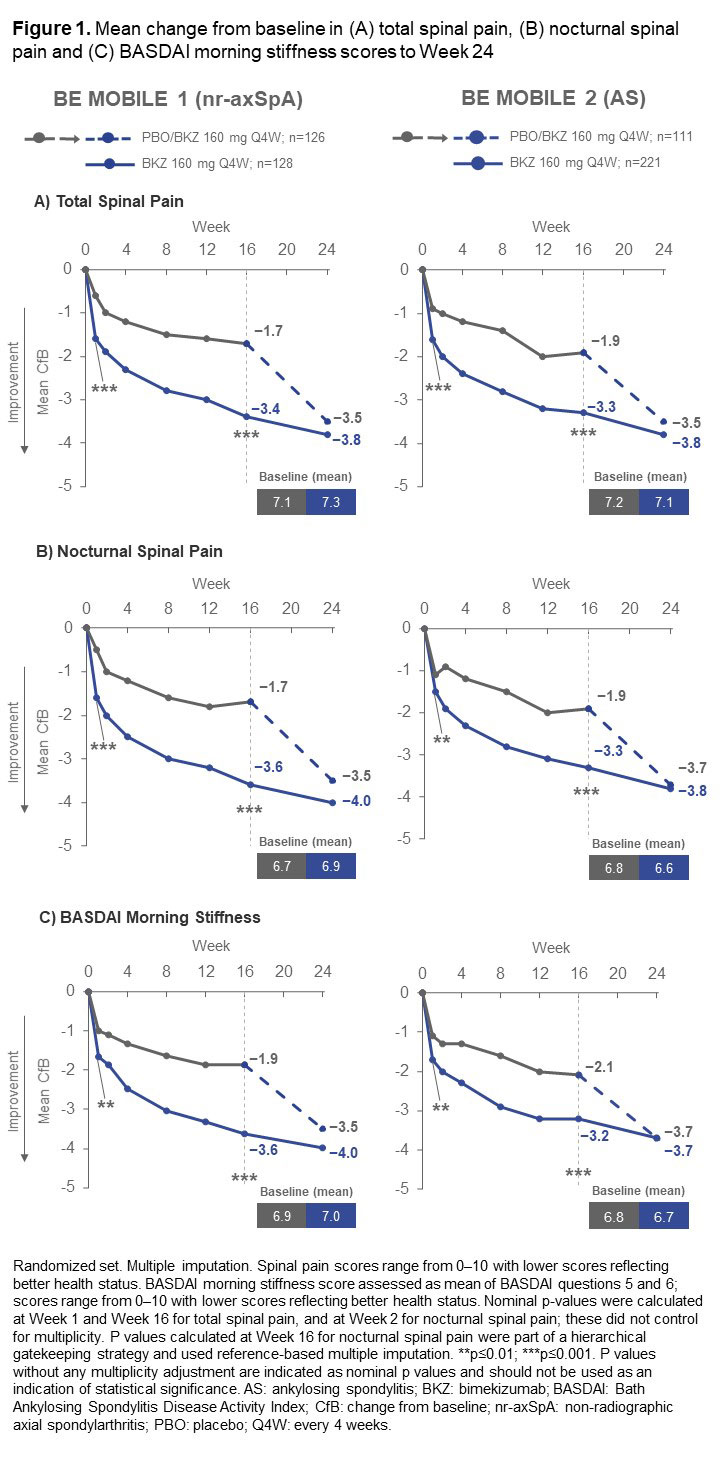
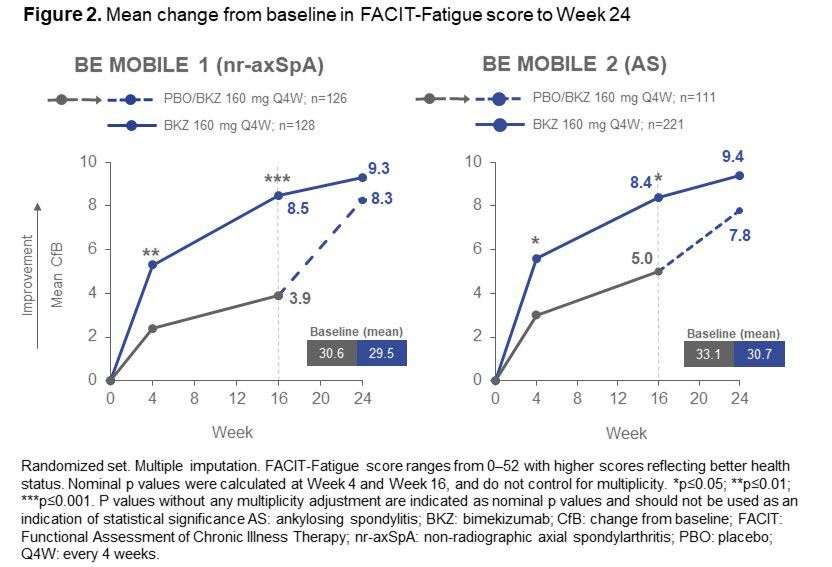
Results: 254 pts with nr-axSpA (BKZ: 128; PBO: 126) and 332 with AS (BKZ: 221; PBO: 111) were randomized; 94.5% and 94.3% completed to Wk 24, respectively. Across both studies, mean BL scores for all reported outcomes indicated high symptom severity (Figure 1, 2).
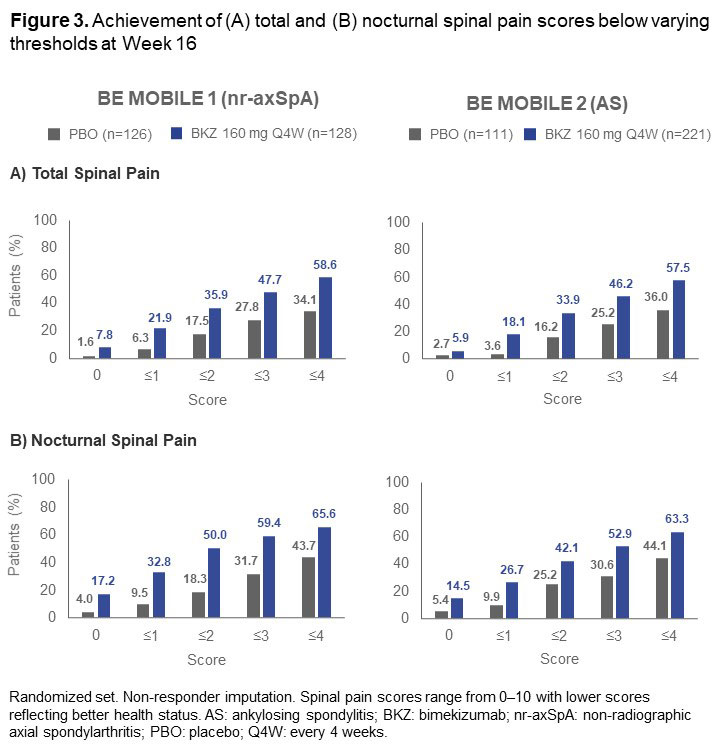
A greater proportion of both nr-axSpA and AS pts treated with BKZ vs PBO achieved lower total and nocturnal spinal pain scores at Wk 16 (Figure 3). Pts treated with BKZ also achieved reductions from BL in mean total spinal pain, nocturnal spinal pain, and BASDAI morning stiffness scores to Wk 24, with separation from PBO observed at the first post-BL assessment (Wk 1; Figure 1). Responses at Wk 24 for pts who switched from PBO to BKZ at Wk 16 approached those seen in BKZ-randomized pts.
Similarly, at Wk 16 a higher proportion of pts achieved ≥4-point improvement in FACIT-Fatigue score with BKZ vs PBO (nr-axSpA: 70.3% vs 45.2%; AS: 66.1% vs 49.5%). Improvement in FACIT-Fatigue scores to Wk 24 were also observed across nr-axSpA and AS pts treated with BKZ, with separation from PBO at first post-BL assessment (Wk 4; Figure 2). Among pts who switched from PBO to BKZ at Wk 16, responses at Wk 24 approached those seen in BKZ-randomized pts.
Conclusion: Treatment with BKZ resulted in rapid and clinically relevant improvements in spinal pain, morning stiffness and fatigue in pts with active axSpA regardless of radiographic classification (AS and nr-axSpA) with separation from PBO at first post-BL assessment. These findings emphasize the benefit of BKZ for clinical symptoms which are important to pts and have significant impact on their daily lives.
References: 1. Deodhar A. Ann Rheum Dis 2022;81:772–3; 2. van der Heijde D. Ann Rheum Dis 2022;81:12–3; 3. Strand V. J Clin Rheumatol 2017;23:383–91.
To cite this abstract in AMA style:
Mease P, Deodhar A, Dougados M, Dubreuil M, Magrey M, Marzo-Ortega H, Rudwaleit M, de la Loge C, Ellis A, Fleurinck C, Oortgiesen M, Taieb V, Gensler L. Bimekizumab Improves Key Patient Reported Symptoms of Axial Spondyloarthritis Including Spinal Pain and Fatigue: Results from Two Phase 3 Studies [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-improves-key-patient-reported-symptoms-of-axial-spondyloarthritis-including-spinal-pain-and-fatigue-results-from-two-phase-3-studies/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-improves-key-patient-reported-symptoms-of-axial-spondyloarthritis-including-spinal-pain-and-fatigue-results-from-two-phase-3-studies/