Session Information
Date: Monday, November 8, 2021
Title: Abstracts: SLE – Treatment: New Agents, Old Agents (1458–1463)
Session Type: Abstract Session
Session Time: 4:15PM-4:30PM
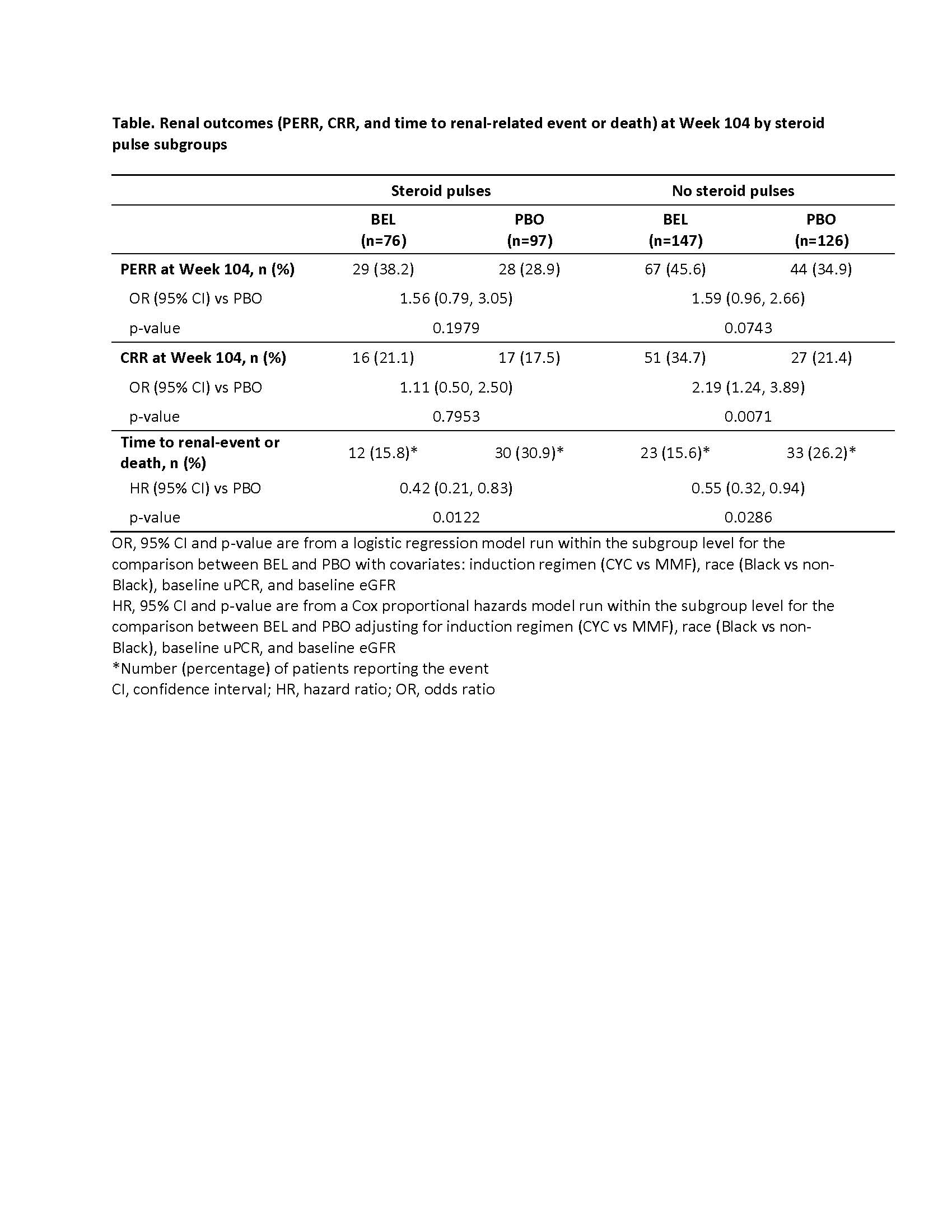
Background/Purpose: In the BLISS-LN trial (GSK Study BEL114054; NCT01639339), the administration of belimumab (BEL), a B-lymphocyte stimulator antagonist, resulted in improved renal outcomes in patients with active lupus nephritis (LN) compared with standard therapy alone.1 The aim of this analysis was to evaluate the effects of BEL on renal outcomes in patients with or without intravenous (IV) steroid pulses during induction therapy for LN in the BLISS-LN trial.
Methods: This post hoc analysis assessed patients from the BLISS-LN trial, a Phase 3, randomized, double-blind, 104-week study of adults with active LN. Patients were administered with either IV BEL 10 mg/kg, or placebo (PBO), plus standard therapy that consisted of oral glucocorticoids and either cyclophosphamide (CYC) for induction followed by azathioprine (AZA) for maintenance, or mycophenolate mofetil (MMF) for both induction and maintenance. At the investigator’s discretion, 1 to 3 IV pulses of methylprednisolone, 500 to 1000 mg each, could be administered during induction. The primary endpoint was Primary Efficacy Renal Response (PERR=urine protein:creatinine ratio [uPCR] ≤0.7; estimated glomerular filtration rate [eGFR] no more than 20% below preflare value or ≥60 ml/min/1.73 m2; no rescue therapy) at Week 104. Other endpoints were Complete Renal Response (CRR=uPCR < 0.5; eGFR no more than 10% below preflare value or ≥90 ml/min/1.73 m2; no rescue therapy) at Week 104, and time to a renal-related event (end-stage kidney disease, doubling of serum creatinine, increased proteinuria and/or impaired renal function, renal disease–related treatment failure) or death. The current analysis compared subgroups of patients who received BEL or PBO with and without IV steroid pulses at induction.
Results: In total, 76 (34.1%) of the 223 patients administered with BEL and 97 (43.5%) of the 223 patients administered with PBO received IV steroid pulses at induction. At Week 104, patients who received BEL, with or without IV steroid pulses at induction, had greater proportions of responders for both PERR and CRR compared with PBO (Table). A higher proportion of patients without, versus those with steroid pulses at induction, achieved PERR and CRR at Week 104, in both BEL and PBO groups. Risk of a renal-related event or death was lower at any time with BEL versus PBO, with or without IV steroid pulses at induction (Table).
Conclusion: The addition of BEL to standard therapy for the treatment of active LN improved renal outcomes compared with PBO in patients with or without IV steroid pulses during induction therapy. Proportions of responders were generally higher in subgroups that did not receive steroid pulses at induction across both BEL and PBO groups. While one might postulate that those who received IV steroid pulses had more active kidney disease and might have been less responsive to therapy, additional analyses of steroid usage and correlates with LN class and severity are needed to address this question.
Funding: GSK
Role of the Study Sponsor: GSK was involved in study design, collection, analysis and interpretation of data, and publication development.
Reference:
1Furie R, et al. N Engl J Med 2020;383(12):1117–28
To cite this abstract in AMA style:
Furie R, Houssiau F, Lightstone L, Yu X, Weinmann-Menke J, Tanaka Y, Jones-Leone A, Gonzalez-Rivera T, Gilbride J, Madan A, Green Y, Roth D. Belimumab Improves Renal Responses in Patients with or Without Steroid Pulses During Induction Therapy for Lupus Nephritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/belimumab-improves-renal-responses-in-patients-with-or-without-steroid-pulses-during-induction-therapy-for-lupus-nephritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/belimumab-improves-renal-responses-in-patients-with-or-without-steroid-pulses-during-induction-therapy-for-lupus-nephritis/