Session Information
Date: Monday, October 27, 2025
Title: (1088–1122) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Large, multi-center efforts are needed to optimally study rheumatic immune-related adverse events (rh-irAEs) from immune checkpoint inhibitor (ICI) use. We aimed to characterize the baseline clinical features of patients enrolled in the Rheumatology Adverse Events Due to Immunotherapy Observational Studies (RADIOS) consortium and examine the severity of rh-irAE at presentation.
Methods: RADIOS is a prospective multi-institutional, national effort enrolling patients with rh-irAEs from 12 academic centers in the US. The consortium began enrolling in 2015 at the initial site and 2022 at additional sites. Patients were included if they were diagnosed by their rheumatologist as having developed a rh-irAE. Descriptive statistics were used to summarize the cohort’s demographics (including age, sex, race, ethnicity), cancer characteristics, and ICI use at enrollment. We evaluated the type of rh-irAE the patient had as well as the Common Terminology Criteria for Adverse Events (CTCAE) grade of the adverse event. Among patients with ICI-induced inflammatory arthritis (ICI-IA), clinical disease activity index (CDAI) was obtained and mean (standard deviation) was calculated.
Results: As of April 1, 2025, the consortium had enrolled 671 patients with a rh-irAE with a mean age of 63.6 years (SD 12.7), of whom 336 were female (50.1%); 580 (86.4%) patients identified as White and 6% as Hispanic. ICI-IA was the most common rh-irAE (n=435, 65%) followed by arthralgia (n=146, 21.8%), PMR (n=69, 10.3%), and myalgia (n=25, 3.7%) (Table 1). Among the cancer types, most patients had melanoma (n=204, 30.5%), followed by non-small cell lung cancer (n=100, 14.9%); 439 patients had received PD-1 inhibitors alone as treatment; 188 patients had anti-CTLA-4/PD-1 combination therapy (Table 2). Average duration of ICI treatment tended to be shorter in those who received combination therapies (ipilimumab/nivolumab and nivolumab/relatilmab) than in those who received anti-PD-1/PD-L1 monotherapy. Among patients with ICI-IA and baseline CDAI available (n=315), the mean score was 16.1 (SD 10.8), indicating most had moderate disease activity. Maximum CTCAE grade of rh-irAE at enrollment for each irAE type is shown in Figure 1. Patients with ICI-IA, PMR, and sicca syndrome most commonly had grade 2 severity while in myositis grade 3 severity was most common.
Conclusion: ICI-IA was the most common rh-irAE in RADIOS, the largest US-based prospective cohort. Melanoma and NSCLC were the most frequent tumor types, with PD-1 inhibitors being the most used class of ICI. Disease activity for ICI-IA measured by CDAI was moderate on average, and the majority of rh-irAEs were grade 2 (limiting instrumental activities of daily living), except for myositis which tended to have a more severe presentation.
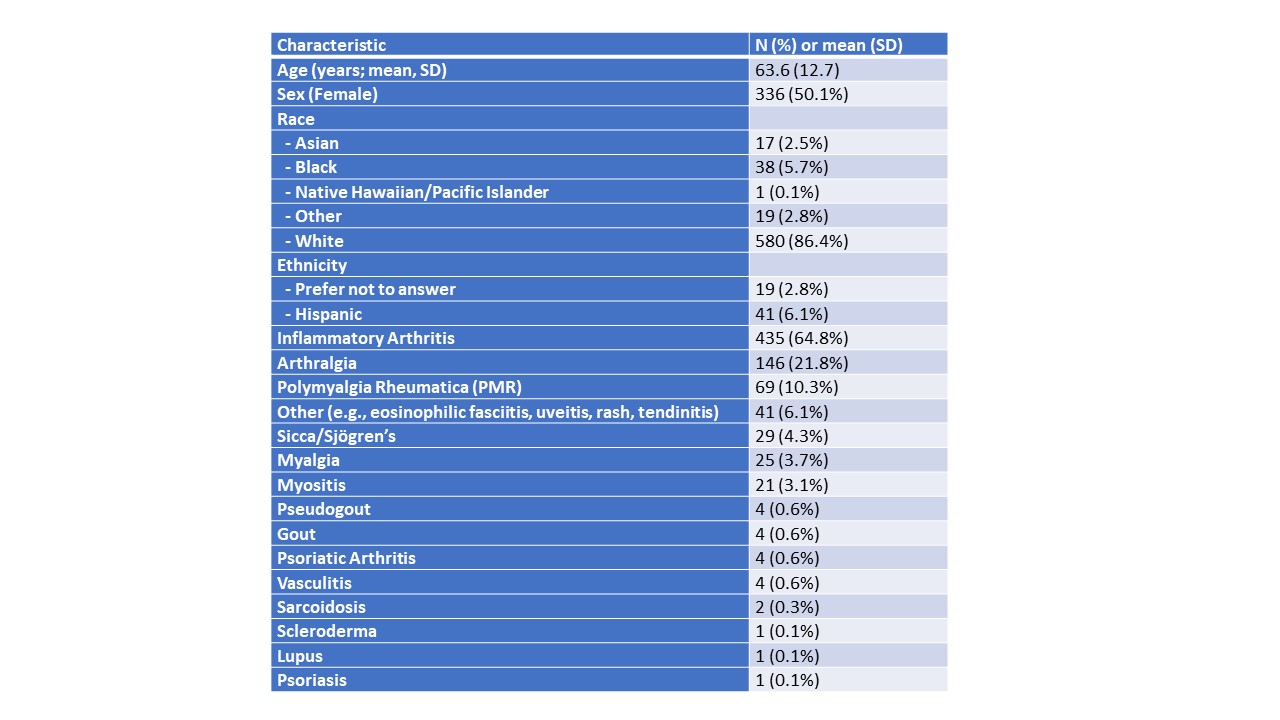 Table 1: Baseline characteristics of patients with rheumatic irAE in RADIOS consortium, 2015-2025 (n=671).
Table 1: Baseline characteristics of patients with rheumatic irAE in RADIOS consortium, 2015-2025 (n=671).
.jpg) Table 2. Cancer type, stage, and ICI exposure at baseline rheumatology visit
Table 2. Cancer type, stage, and ICI exposure at baseline rheumatology visit
.jpg) Figure 1: Common Terminology Criteria for Adverse Events (CTCAE) grade for rh-irAEs at time of enrollment to RADIOS. Grade 1 events are mild and/or self-limited, grade 2 events limit instrumental activities of daily living, grade 3 events are severe and limit self-care activities of daily living, and grade 4 events are severe, potentially life threatening, and/or require hospitalization.
Figure 1: Common Terminology Criteria for Adverse Events (CTCAE) grade for rh-irAEs at time of enrollment to RADIOS. Grade 1 events are mild and/or self-limited, grade 2 events limit instrumental activities of daily living, grade 3 events are severe and limit self-care activities of daily living, and grade 4 events are severe, potentially life threatening, and/or require hospitalization.
To cite this abstract in AMA style:
Cappelli L, Singh N, Abdel-Wahab N, Bass A, Braaten T, Calabrese C, Ghosh N, Katsumoto T, Kim S, Kohler M, Meara A, Reid P, Sparks J, Suarez-Almazor M, Bingham C, Shah A. Baseline Data on Rheumatic Immune-related Adverse Events from the Largest Prospective Multicenter US Consortium [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/baseline-data-on-rheumatic-immune-related-adverse-events-from-the-largest-prospective-multicenter-us-consortium/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-data-on-rheumatic-immune-related-adverse-events-from-the-largest-prospective-multicenter-us-consortium/
