Session Information
Date: Sunday, November 17, 2024
Title: Abstracts: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science I
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: The aim of the study was to assess the effects of baricitinib, a JAK1/2 inhibitor, following 24 weeks of active treatment on disease activity in adult patients with Idiopathic Inflammatory Myopathy (IIM) (ISRCTN 30448031).
Methods: Patients with confirmed dermatomyositis (DM) or polymyositis (PM), as per 2017 EULAR/ACR classification criteria for IIM (Lundberg IE et al, Ann Rheum Dis. 2017 Dec;76(12):1955-1964), with persisting active disease after treatment with glucocorticoids and/or ≥ one disease modifying drug for ≥ 3 months, were enrolled 1:1 in a randomized treatment delayed-start design trial within the UK, to receive either 24 weeks of active treatment from randomisation with 12 weeks follow-up (immediate start arm), or 12 weeks of standard of care followed by 24 weeks active treatment (delayed-start arm).
The primary outcome was the clinical response across treatment arms after 24 weeks of active treatment, defined as minimal improvement according to the 2016 ACR/EULAR response criteria for adult PM and DM (Aggarwal R, et al. Arthritis Rheumatol. 2017 May;69(5):898-910).
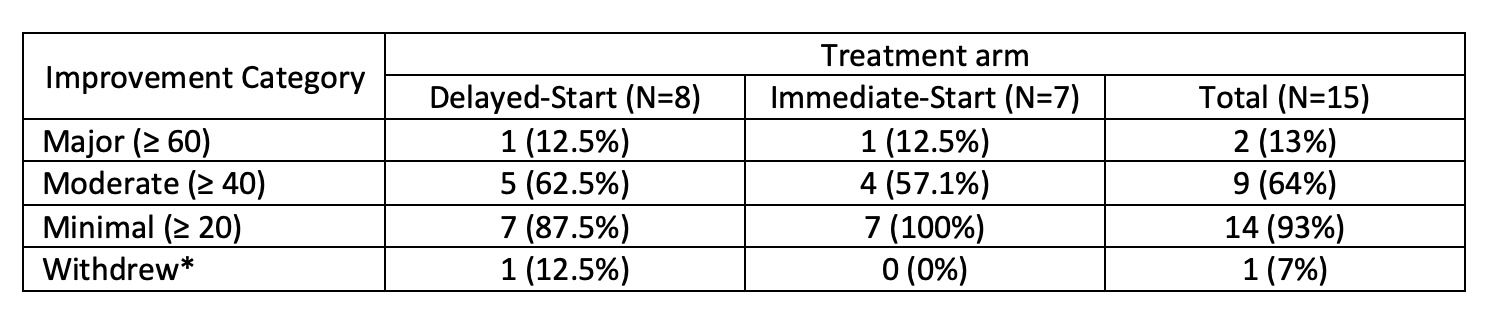
Results: Among 15 randomized patients (mean age 43.2 [11.6 SD]; 13 DM, 2 PM; 11 female, 4 male), 14 patients completed the study (baseline to 36 weeks); one patient withdrew from the trial prior to the primary outcome visit. Using intention to treat analysis, 14/15 (93%) achieved the primary outcome of at least minimal clinical response (Total Improvement Score >20) at 24 weeks post-active treatment (95% exact CI 0.68 to 1.00).
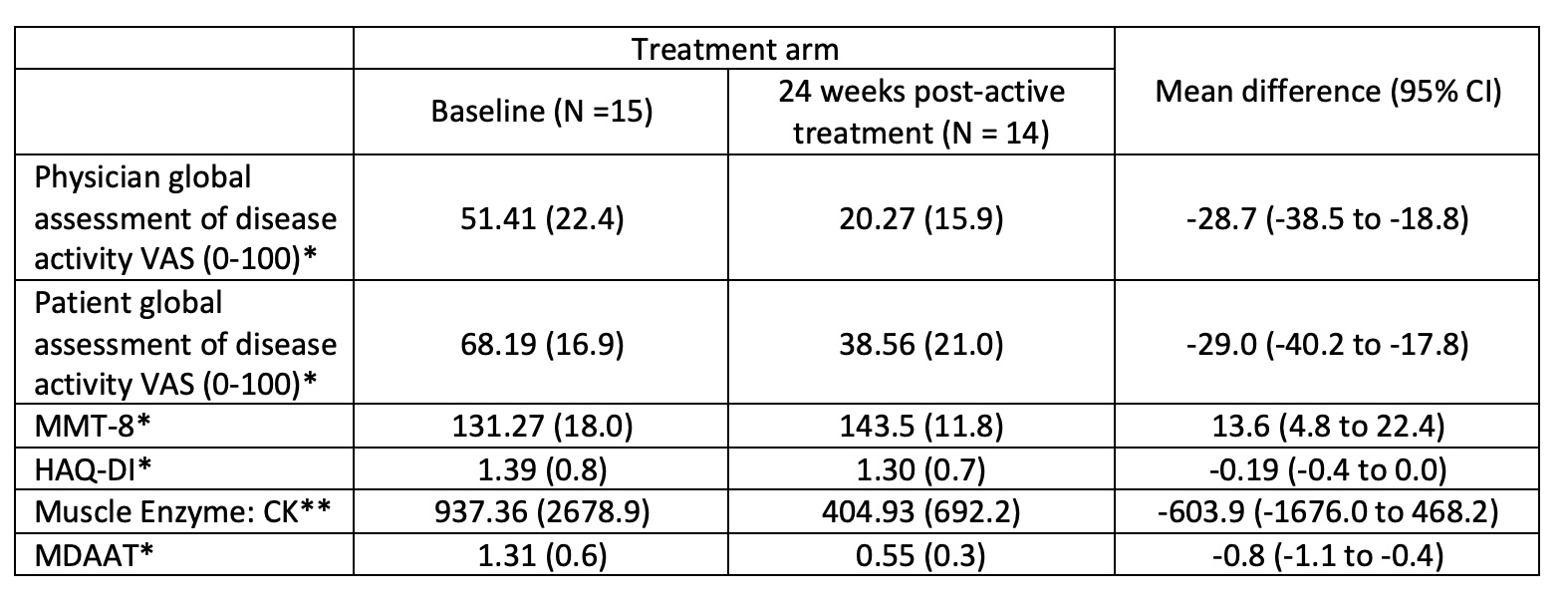
Regarding secondary outcomes, 9/15 (60%) achieved moderate clinical response (Table 2). There was no significant difference in response rates between the immediate- and delayed-start arms for those who achieved at least moderate clinical response at 24 weeks post-active treatment (0.14, 95% CI [-0.35 to 0.64]). After active treatment for 24 weeks, the immediate-start arm improved muscle strength, as assessed using the bilateral manual muscle test (MMT)-8, by 10.1 points, and the delayed-start arm by 17 (mean difference -6.9, -25.8-12.0). Regarding individual core set measures at week 24, significant differences from baseline were noted for physician/patient VAS, bilateral MMT-8 and extramuscular disease VAS (Table 2, post-hoc analysis). 11/15 (73%) achieved at least minimal clinical response at 12 weeks post-active treatment (95% exact CI 0.45 to 0.92). On post-hoc analysis at 12 weeks from baseline, 7 (100%) immediate-start patients achieved minimal response after active treatment vs. 4 (50%) delayed-start arm patients yet to commence baricitinib (0.5, 0.15-0.85). There were two serious AE requiring hospitalisation (skin laceration, pre-syncope), neither of which was related to the study drug.
Conclusion: Treatment of IIM patients with baricitinib resulted in improved clinical response after 24 weeks with a significant improvement after 12 weeks of active treatment. No significant safety concerns were raised.
*One patient withdrew from the trial prior to the Primary outcome data being collected. The patient attended an early termination visit 10 days prior to Visit 9 (as per Statistical Analysis Plan data cannot be included for Primary outcome analysis). However, for information based on their early termination visit data this patient achieved minimal clinical response.
VAS=visual analogue score; MMT-8=bilateral manual muscle test-8; HAQ-DI=Health Assessment Questionnaire-Disability Index; CK=creatine kinase; MDAAT=Myositis disease activity assessment tool; *assessed in 14/15 patients; **assessed in 13/15 patients.
To cite this abstract in AMA style:
Chinoy h, Krishan A, Sylvestre Y, Lilleker J, Gordon P, Tansley S, Prabu A, Aslam A, Snedden A, Lamb J. Baricitinib in the Treatment of Adult Idiopathic Inflammatory Myopathy: A Randomized, Treatment Delayed-Start Clinical Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/baricitinib-in-the-treatment-of-adult-idiopathic-inflammatory-myopathy-a-randomized-treatment-delayed-start-clinical-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baricitinib-in-the-treatment-of-adult-idiopathic-inflammatory-myopathy-a-randomized-treatment-delayed-start-clinical-trial/