Session Information
Date: Saturday, November 12, 2022
Title: RA – Animal Models Poster
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: In clinical trials for the treatment of rheumatoid arthritis, baricitinib was shown to significantly improve pain scores compared with anti-TNF inhibitors. This result suggests that baricitinib may regulate pain independently of arthritis improvement. Here, we aimed to reveal the mechanism underlying the regulation of pain by baricitinib, using the collagen antibody-induced arthritis (CAIA) mouse model.
Methods: We intragastrically administered baricitinib, celecoxib, or vehicle once per day to CAIA mice; we also administered vehicle to control mice without CAIA (Control group). We then analyzed the four groups. We evaluated the progression of arthritis by visual scoring, inflammatory pain by the grip strength test, and allodynia by the von Frey test. Dorsal root ganglion (DRG) and spinal cords were harvested on day 8 or 14 following induction of arthritis, to evaluate the expression of the pain-related molecules. We used RNA sequencing (RNAseq) and quantitative PCR to analyzed gene expression in the DRG. We used immunohistochemistry to examine activation of glial cells in the spinal cord.
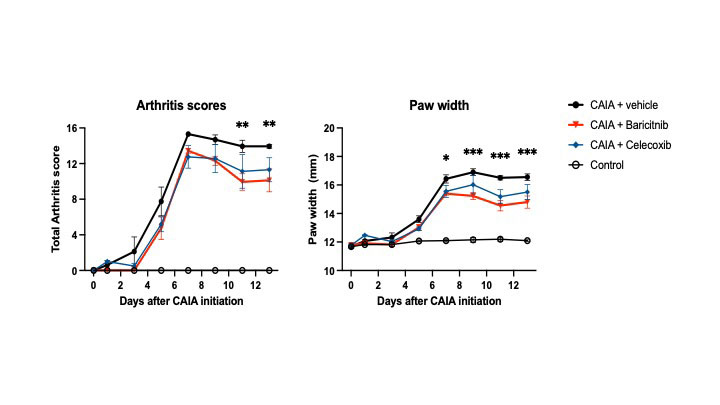
Results: In CAIA mice with vehicle treatment (CAIA group), the progression of arthritis and decrease in grip strength were severe around day 8 and gradually attenuated thereafter, while allodynia peaked around day 14 and was sustained. Arthritis and grip strength were equivalently improved in the baricitinib and celecoxib groups compared with the CAIA group. However, allodynia was markedly suppressed to a level similar to that seen in the Control group by baricitinib treatment but not by celecoxib treatment. RNAseq showed that the expression of genes related to inflammation and the immune response increased in the DRG of the CAIA group at day 8, compared with the Control group. The upregulated genes were decreased in the baricitinib group. Notably, the increased expression of IL-6-JAK-STAT3 pathway-related genes by CAIA was attenuated by baricitinib treatment. Expression of Socs3, a representative downstream gene of the JAK/STAT pathway, was significantly suppressed in the baricitinib group. The activation of glial cells, particularly astrocytes, was significantly more inhibited in the spinal cords of the baricitinib group than in those of the celecoxib group at day 14.
Conclusion: Only baricitinib treatment suppressed allodynia in CAIA mice, while both baricitinib and celecoxib improved arthritis and inflammatory pain. Baricitinib also suppressed gene expression related to the inflammation and immune responses in the DRG of CAIA mice and the subsequent activation of astrocytes in the spinal cord. Baricitinib may contribute to the amelioration of residual allodynia in rheumatoid arthritis via modulating the IL6-JAK-STAT3 pathway in the DRG, as well as that of arthritis itself.
n = 4, two-way ANOVA followed by Tukey’s multiple comparisons test. Values are the means ± SEM
*p<0.05, ** p<0.01, *** p<0.001 (CAIA + Baricitinib vs CAIA)
To cite this abstract in AMA style:
Makabe K, Omata Y, Okada H, Chijimatsu R, Terashima A, Yano F, Tanaka S, Saito T. Baricitinib Ameliorates Residual Neuropathic Pain in Collagen Antibody-Induced Arthritis Mice by Suppressing Inflammation of the Dorsal Root Ganglion [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/baricitinib-ameliorates-residual-neuropathic-pain-in-collagen-antibody-induced-arthritis-mice-by-suppressing-inflammation-of-the-dorsal-root-ganglion/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baricitinib-ameliorates-residual-neuropathic-pain-in-collagen-antibody-induced-arthritis-mice-by-suppressing-inflammation-of-the-dorsal-root-ganglion/