Session Information
Date: Saturday, November 6, 2021
Title: Abstracts: Systemic Sclerosis & Related Disorders – Clinical (0496–0501)
Session Type: Abstract Session
Session Time: 4:00PM-4:15PM
Background/Purpose: Treatment of dcSSc is evolving, with limited information on relative efficacies of different immunosuppressive therapies (IST). Our hypothesis was that patients on MMF have better efficacy outcomes than patients on other IST or no IST, in a clinical trial context.
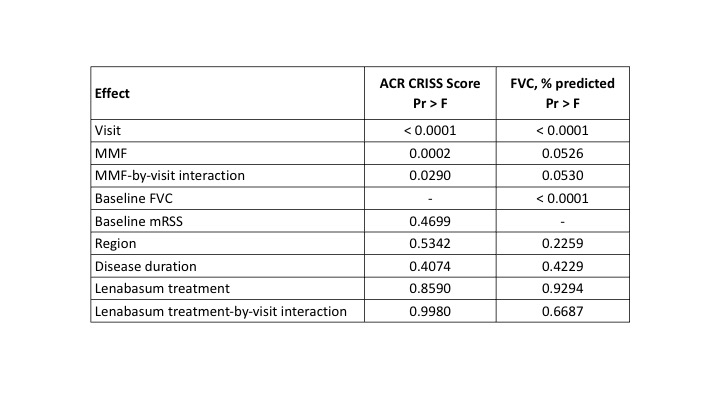
Methods: Data were analyzed from a 1-year Phase 3 double-blind, placebo-controlled study of lenabasum in subjects with dcSSc ≤ 6 years duration. Background IST (bIST) were allowed in doses that were stable for ≥ 8 weeks before Screening. Enrolling sites were mostly academic specialty clinics for patients with SSc. All cohorts were combined for analyses, because the primary efficacy endpoint (ACR CRISS score) was not met. Subjects were categorized by bIST use: None, MMF, Steroids (≤ 10 mg/day oral prednisone or equivalent), Methotrexate (MTX), and All Other IST. Baseline demographics, disease characteristics, and efficacy outcomes were determined and impact of MMF on certain outcomes was determined in mixed model repeated measures analyses (MMRM) with visit, MMF use, MMF-by-visit interaction, baseline mRSS score, disease duration, region, lenabasum treatment, and lenabasum treatment-by-visit interaction as fixed effects.
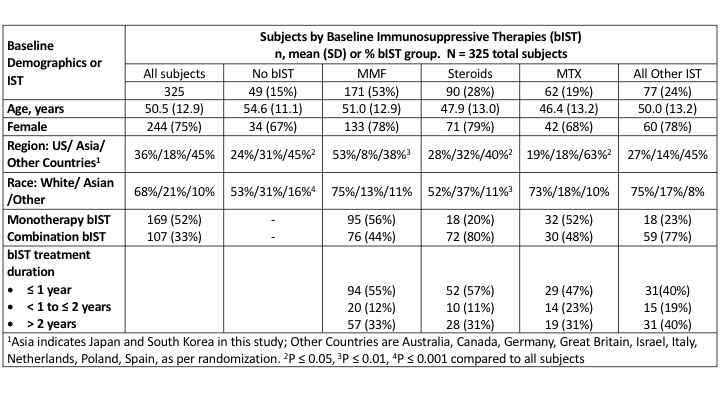
Results: 276/325 (85%) subjects used bIST (Table 1). MMF was used in about twice as many subjects as Steroids (53% vs. 28%), followed in descending order by: MTX (19%); and All Other bIST, comprised of hydroxychloroquine (11%); tocilizumab, immunoglobulins, and azathioprine (4% each); and chloroquine, cyclophosphamide, abatacept, tacrolimus, and cyclosporine (≤ 1% each). Subjects treated with different bIST had similar age and sex. Treatment with MMF was more common in the US and White subjects, whereas treatment with Steroids or No bIST was more common in Asia and Asian subjects. Treatment with MTX was more common in Other Countries (mostly Europe). MMF and MTX were used more commonly as monotherapy (56% and 52%), respectively, than Steroids (20%). Treatment duration of bIST (≤ 1 year, >1 – ≤ 2years, > 2 years) did not differ significantly among bIST groups.
Subjects treated with MMF had greater improvement in most efficacy outcomes than subjects treated with MTX or steroids without MMF (Table 2). Visit, use of MMF, and MMF-by-visit interaction had significant effects on ACR CRISS score, whereas baseline mRSS, region, disease duration, lenabasum treatment, and lenabasum treatment-by-visit interaction did not (Table 3). Use of MMF, visit, MMF-by-visit interaction, and baseline FVC % also influenced change in FVC%, whereas the other fixed effects did not.
Conclusion: MMF was the most commonly used bIST in this Phase 3 study of dcSSc subjects. Treatment with MMF during the trial was associated with better outcomes than treatment with MTX or Steroids without MMF. In future dcSSc trials that enroll subjects on bIST, statistical analyses should consider that subjects on placebo added to bIST are, in fact, receiving active concomitant treatment that will confound trial results over time. Differences in proportion of subjects on MMF, other IST, and no IST may also influence results. Efficacy outcomes will need to detect improvement beyond the substantial efficacy already provided by bIST over time.
To cite this abstract in AMA style:
Spiera R, Furst D, Frech T, Kuwana M, Hummers L, Stevens W, Kafaja S, Lee E, Constantine S, Dgetluck N, White B, Denton C. Background Mycophenolate (MMF) Treatment Is Associated with Improved Outcomes in a Phase 3 Trial of Lenabasum in Diffuse Cutaneous Systemic Sclerosis (dcSSc) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/background-mycophenolate-mmf-treatment-is-associated-with-improved-outcomes-in-a-phase-3-trial-of-lenabasum-in-diffuse-cutaneous-systemic-sclerosis-dcssc/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/background-mycophenolate-mmf-treatment-is-associated-with-improved-outcomes-in-a-phase-3-trial-of-lenabasum-in-diffuse-cutaneous-systemic-sclerosis-dcssc/