Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: In the phase 3 ADVOCATE trial, 45.2% and 42.1% of patients in the avacopan and prednisone taper groups, respectively, had active ear, nose, or throat (ENT) manifestations of ANCA-associated vasculitis (either granulomatosis with polyangiitis [GPA] or microscopic polyangiitis [MPA]).1 These proportions dropped to 1.2% (2 patients) at both weeks 26 and 52 in the avacopan group, and to 3.7% (6 patients) at week 26 and 3.0% (5 patients) at week 52 in the prednisone taper group.2 However, the impact of avacopan on other outcomes in patients with ENT manifestations of GPA/MPA is unknown. These findings are presented herein.
Methods: ADVOCATE was a double-blind, double-dummy, active-controlled trial, with patients randomized 1:1 to receive avacopan (30 mg twice daily) or a prednisone taper (60 mg/day tapered to 0 by week 21) on a background of cyclophosphamide (followed by azathioprine or mycophenolate mofetil) or rituximab. In this subgroup post hoc analysis of patients with ENT involvement at baseline based on Birmingham Vasculitis Activity Score, key outcomes were the proportion of patients achieving remission at week 26 and sustaining remission at week 52. Other outcomes were proportion of patients with active ENT manifestations, glucocorticoid (GC) dose (prednisone-equivalent), GC Toxicity Index (GTI), health-related quality of life, and safety.
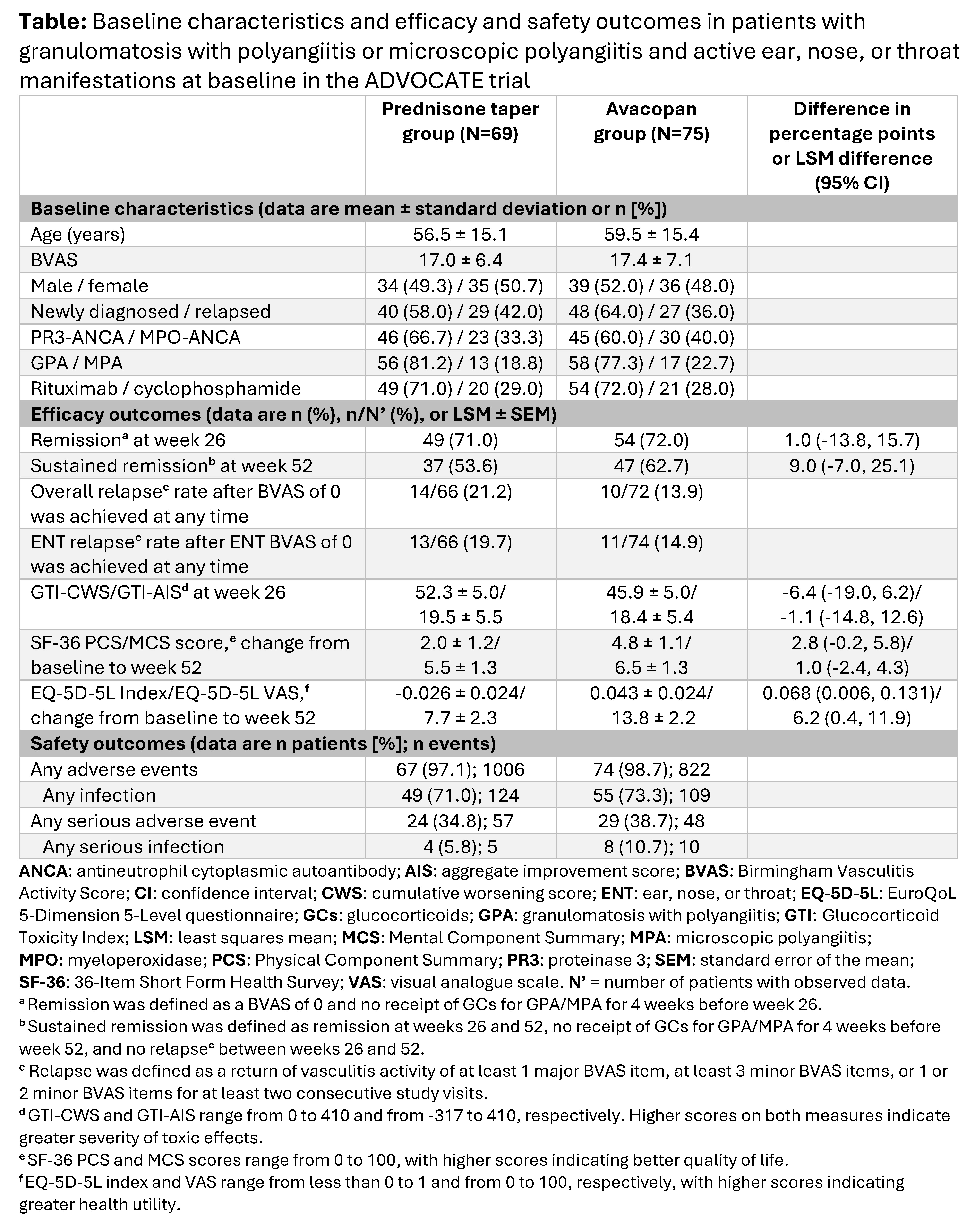
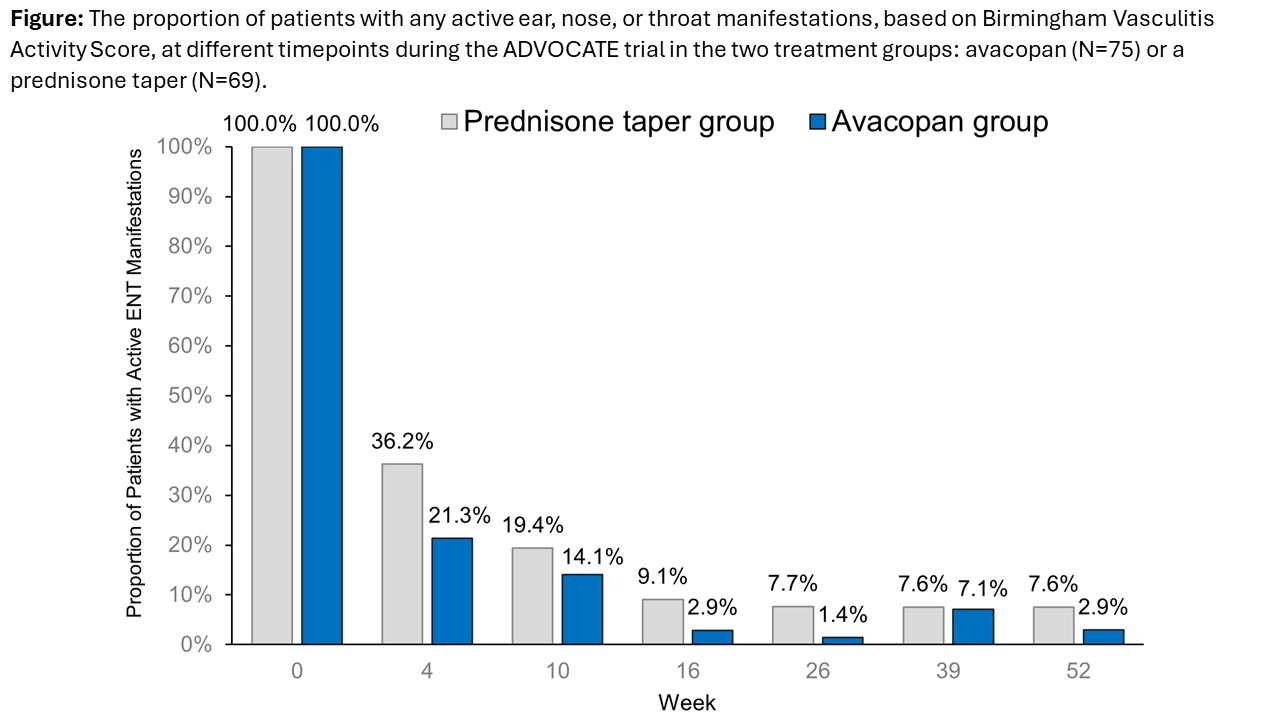
Results: Baseline characteristics of the 144 patients with active ENT manifestations (avacopan, N=75; prednisone taper, N=69) were comparable across treatment groups (Table) The proportion of patients with active ENT manifestations at week 4 decreased more rapidly with avacopan vs a prednisone taper (21.3% vs 36.2%) (Figure). The mean/median total all-source GC dose was 680/500 mg with avacopan vs 1677/1630 mg with a prednisone taper from day 1 to week 4. A similar proportion of patients with avacopan vs a prednisone taper achieved remission at week 26 (72.0% vs 71.0%) while a higher proportion sustained remission at week 52 (62.7% vs 53.6%; difference: 9.0%; 95% CI: -7.0%, 25.1%) (Table). Relapse within the ENT domain was lower with avacopan (14.9%) vs a prednisone taper (19.7%). Both GTI scores at week 26 were lower (more favorable) with avacopan vs a prednisone taper. The improvements from baseline in EuroQoL 5-Dimension 5-Level index and visual analogue scale were greater with avacopan vs a prednisone taper at week 52 (Table). Infections occurred in 55 patients (73.3%; 109 events) in the avacopan group and in 49 patients (71.0%; 124 events) in the prednisone taper group; 1 death (worsening of vasculitis) occurred in the avacopan group (1.3%).
Conclusion: Findings from this post hoc subgroup analysis of ADVOCATE in patients with GPA or MPA and active ENT manifestations at baseline showed that avacopan was associated with faster resolution of ENT manifestations, higher rate of sustained remission, lower relapse rate, lower GC-related toxicity, greater improvement in HRQoL, and similar safety vs a prednisone taper. These data indicate avacopan may be efficacious in patients with ENT involvement of GPA or MPA.
References: 1. Jayne DRW et al. N Engl J Med 2021;384:599-609; 2. Specks U et al. Am J Respir Crit Care Med 2022;205:A4780
To cite this abstract in AMA style:
Spiera R, Lebovics R, Bray S, Gurlin R, Jayne D, Merkel P. Avacopan versus a Prednisone Taper in Patients with ANCA-Associated Vasculitis and Ear, Nose, or Throat Involvement in a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/avacopan-versus-a-prednisone-taper-in-patients-with-anca-associated-vasculitis-and-ear-nose-or-throat-involvement-in-a-phase-3-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/avacopan-versus-a-prednisone-taper-in-patients-with-anca-associated-vasculitis-and-ear-nose-or-throat-involvement-in-a-phase-3-trial/