Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Avacopan is approved in the US for use in adults with severe active GPA or MPA alongside background immunosuppression. Efficacy and safety data on avacopan in the real-world setting are limited.
Methods: This ongoing retrospective cohort study included patients who were prescribed avacopan between 12/1/21-4/1/24 for GPA or MPA in a large healthcare system. The index date was the date of first avacopan prescription. Data were extracted by manual review and structured data from the electronic health records and were included from 12 months prior to index date through 6 months after avacopan initiation (among initiators). Complete remission was defined as a Birmingham Vasculitis Activity Score (BVAS v3) of 0 at month 6 and no glucocorticoids (GCs) after month 5. Outcomes at 6 months among initiators in the overall cohort and in subgroups with ENT, pulmonary, or kidney involvement are reported.
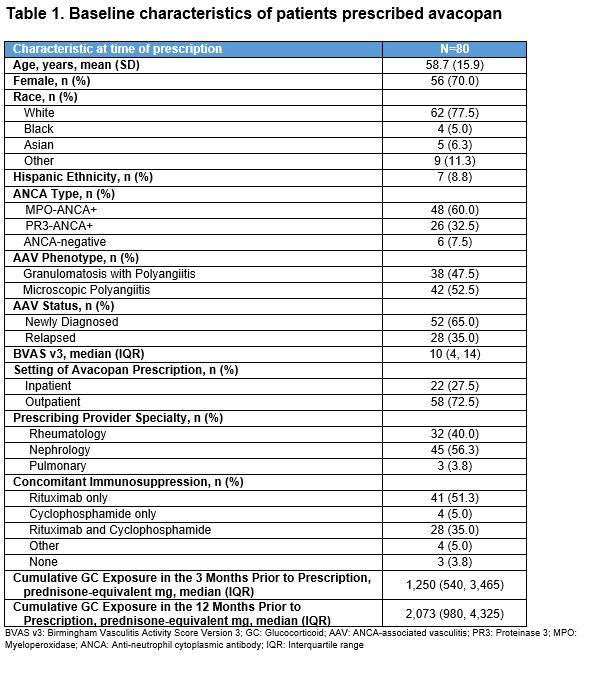
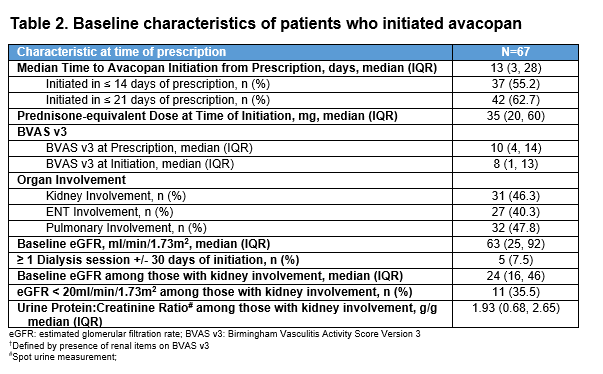
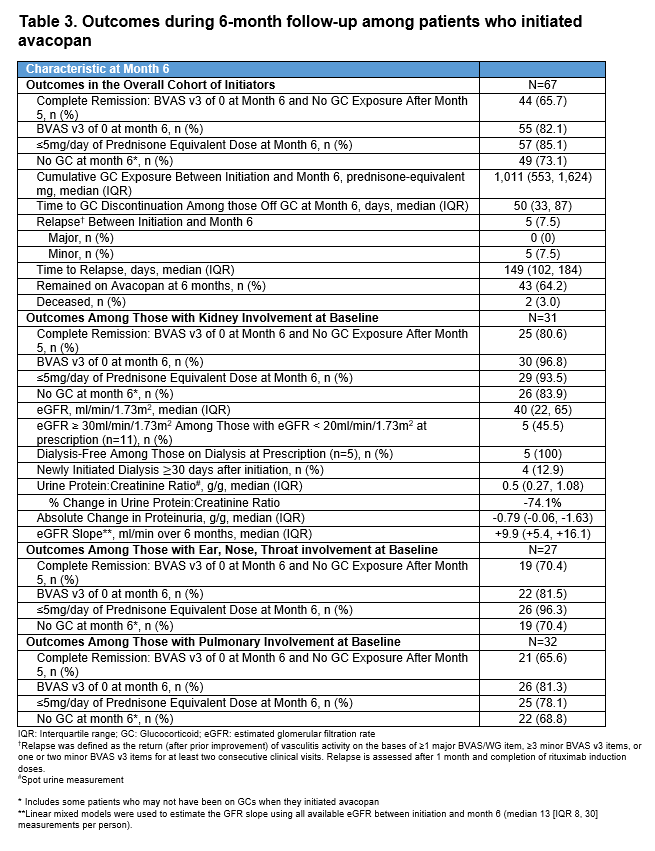
Results: At the time of avacopan prescription in the first 80 patients (Table 1), the mean age was 59 years, and most were female (70%), newly diagnosed (65%), MPO-ANCA+ (60%), and had MPA (53%). Nephrologists were the most frequent prescribers (56%). Of these 80 patients, 67 (84%) initiated avacopan. At initiation (Table 2), 31 (46%) had kidney involvement based on BVAS items (median estimated glomerular filtration rate [eGFR] 24 ml/min/1.73m2; urine protein-to-creatinine ratio [UPCR] 1.9 g/g). At month 6, among the 67 initiators, median cumulative GC exposure from months 0-6 was 1,011 prednisone-equivalent mg, 82% had a BVAS of 0, 73% were on no GC at month 6, and 66% achieved complete remission (Table 3). All 5 patients with ≥1 dialysis session ±30 days of avacopan initiation were off dialysis by month 6; four additional patients newly initiated dialysis ≥30 days after starting avacopan. By month 6, UPCR was 0.5 g/g. Of the 11 with an eGFR < 20 ml/min/1.73m2 at initiation, 5 had an eGFR ≥30 ml/min/1.73m2 by month 6. Among those with kidney (n=31), ENT (n=27), or pulmonary (n=32) involvement at avacopan initiation, the rates of complete remission were 80.6%, 70.4%, and 65.6%, respectively. In the 35 patients who stopped GCs by month 6 (on GCs at avacopan initiation), the median time to GC discontinuation was 50 days. At month 6, 43/67 (64%) remained on avacopan with the rest stopping at or before month 6; 8 (12%) completed a planned 6-month course; 9 (13%) discontinued due to adverse events including transaminitis (n=2), rash (n=2), GERD (n=1), paresthesias (n=1), pruritis (n=1), dizziness (n=1), hair thinning (n=1), and flare (n=1); other reasons were cost (n=1) and self-discontinuation (n=3). Two died before month 6: one with interstitial lung disease died from COVID-19 32 days after avacopan initiation and one from disseminated aspergillosis 23 days after avacopan initiation.
Conclusion: In this real-world cohort of patients with GPA or MPA, avacopan was prescribed for individuals with a spectrum of GPA and MPA manifestations. A large proportion of users, overall and across kidney, ENT, and pulmonary subgroups, discontinued GCs by month 5 and achieved complete remission. Among those with kidney involvement, significant reductions in proteinuria and improvement in the eGFR were observed by month 6.
To cite this abstract in AMA style:
Patel N, Fernandes A, King A, Srivatsan S, Johnson C, Williams Z, Negron M, Bolden C, Lin M, Jiang B, Oh S, Trimpe D, Inguva S, Wallace Z. Avacopan for Granulomatosis with Polyangiitis (GPA) and Microscopic Polyangiitis (MPA) in a Real-World Setting [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/avacopan-for-granulomatosis-with-polyangiitis-gpa-and-microscopic-polyangiitis-mpa-in-a-real-world-setting/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/avacopan-for-granulomatosis-with-polyangiitis-gpa-and-microscopic-polyangiitis-mpa-in-a-real-world-setting/