Session Information
Date: Sunday, November 7, 2021
Title: Pediatric Rheumatology – Clinical Poster II: SLE, JDM, & Juvenile Scleroderma (0764–0785)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
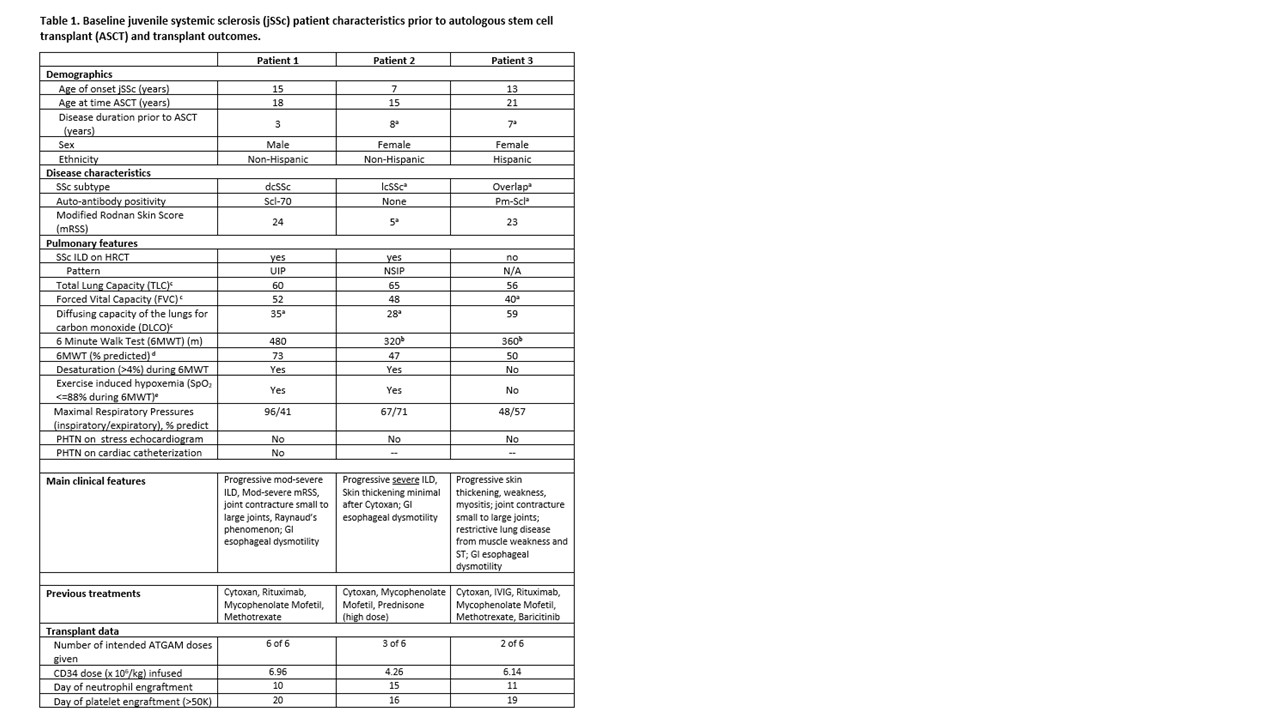
Background/Purpose: Juvenile-onset systemic sclerosis (jSSc) is a rare autoimmune disease associated with life-threatening multi-organ inflammation and fibrosis. As in adults, jSSc organ involvement includes vascular, cutaneous, gastrointestinal, pulmonary, and musculoskeletal systems. Treatment options are limited for aggressive and recalcitrant SSc, however advances in adult SSc therapies support Autologous Stem Cell Transplant (ASCT) as a safe option to reset the immune system and prevent eminent decline. Our center has developed reduced intensity conditioning protocols for both pediatric and adult onset SSc using CD34-selected peripheral blood ASCT, including our Immune Transplant and Therapy Center (ITTC) protocol (ClinicalTrials.gov Identifier: NCT03630211). We report initial safety and clinical response of three jSSc patients who received ASCT at our center (Table 1) with comorbidities too advanced even for our trial.
Methods: Patients with severe and progressive disease referred to our center were evaluated by pediatric rheumatologist (KT) and referred to BMT (PS, JB) if deemed to be refractory to standard clinical care. A dedicated multi-disciplinary team evaluated the patients (Rheum, BMT, Cards, Pulm, GI) to determine baseline status and eligibility for an existing protocol (ITTC) or an Investigational New Drug (IND) application if more suitable. After IRB approval and consent, patients underwent ASCT with standardized safety, clinical outcome, and patient reported measures collected at baseline (pre-ASCT), 6, and 12 months-post ASCT.
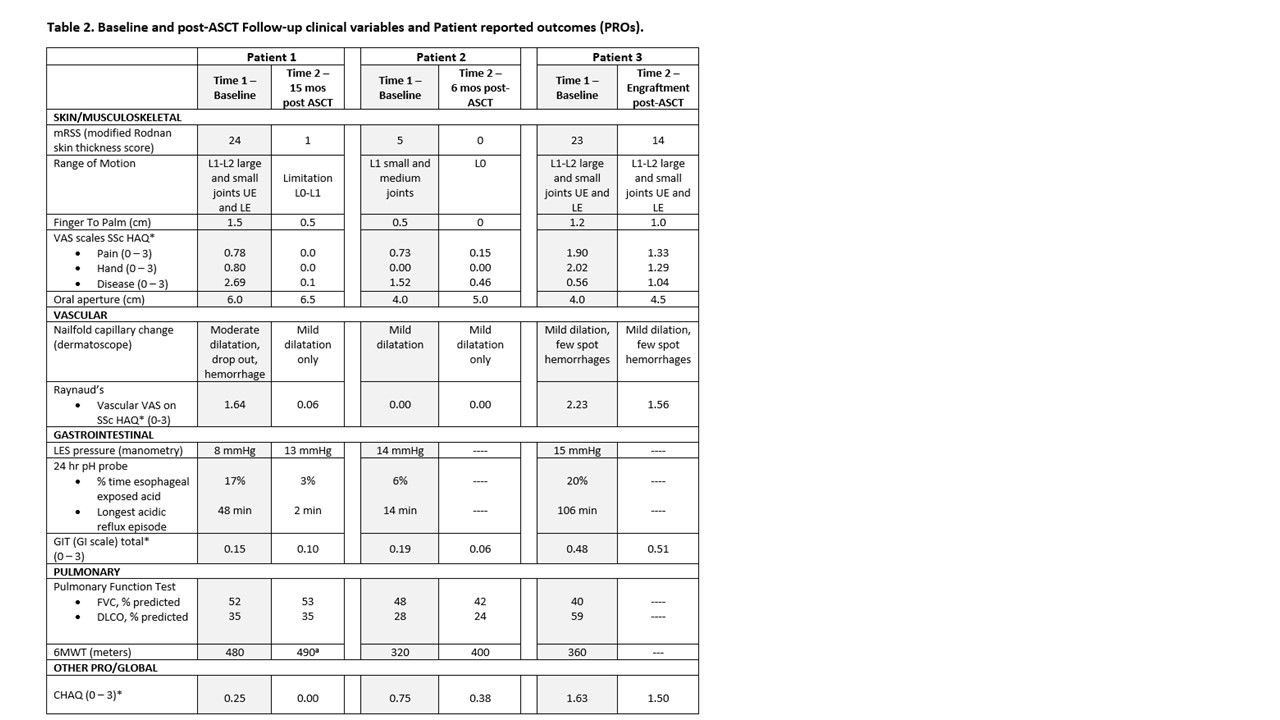
Results: Three jSSc patients were eligible to receive ASCT by the multi-disciplinary team (Table 1); all failed to meet ITTC eligibility criteria due to poor respiratory function (FVC < 45% and/or DLCO < 40%; and 6MWT < 400m; see Table 1) and had individual INDs approved for ASCT. All consisted of Cyclophosphamide 120 mg/kg, total body irradiation (6 Gy in 3 fractions with liver and kidney shielding) and ATGAM. At time points of 15 months-post ASCT, 6 months-post ASCT, and engraftment for patients 1, 2, and 3, respectively, only patient 2 had a major safety concern, a significant ATGAM reaction requiring a brief ICU stay for increased supportive needs, with protocol resumption within 48 hours. Patient 3 had a milder ATGAM reaction. There have been no serious infections or organ dysfunction to date. All patients showed improvement in clinical outcomes of mRSS, range of motion, physical functioning (HAQ), and several PRO domains including dyspnea, Raynaud’s phenomenon, and gastrointestinal symptoms (Table 2).
Conclusion: Our preliminary data supports the safety and efficacy of ASCT with CD34-Selected Peripheral Blood Stem Cells in jSSc. Two subjects had 7+ years of duration: one with more limited skin disease, and the other with overlap disease with myositis, both with positive outcomes. Expansion of ASCT eligibility to include children, adolescents and young adults and those with clinical features beyond classic early diffuse cutaneous SSc should be considered while maintaining an acceptable safety profile.
a Clinical variables that would have failed adult SSc entry criteria ASCT trials: Autologous Stem Cell Transplantation International Trial (ASTIS) (van Laar et al JAMA 2014; 311(24)) and Scleroderma: Cyclophosphamide or Transplantation (SCOT) (Sullivan et al NEJM 2018; 378(11)).
b Additional clinical variables that would have failed adult SSc entry criteria ITTC trial (6MWT >400).
c Values represent percent predicted based on GLI2012 prediction equations for FVC and DLCO Quanjer et al. Eur Respir J
2012;40(6), and Rosenthal et al. Thorax 1993;48(8) for TLC.
d Percent predicted based on Geiger et. al. J Pediatrics 2007; 150(4), pediatric prediction equations.
e SpO2 was monitored continuously during 6MWT via forehead probe.
Abbreviations:
dcSSc (diffuse cutaneous systemic sclerosis); lcSSc (limited cutaneous systemic sclerosis); Scl-70 (scleroderma-70 anti-topoisomerase antibody); PM-Scl (polymyositis-scleroderma overlap antibody); ILD (interstitial lung disease); PHTN – (pulmonary hypertension)
Abbreviations:
ASCT (autologous stem cell transplant); VAS (Visual Analog Scale); HAQ (Health Assessment Questionnaire); LES (lower esophageal sphincter); GIT (scleroderma gastrointestinal tract instrument); FVC (forced vital capacity); DLCO (diffusing capacity for carbon monoxide); 6MWT (6 minute walk test). GLI2012 prediction equations used for both FVC and DLCO.
* Indicates a patient reported outcome. VAS scale on HAQ, GIT, CHAQ are 0 to 3; higher number reflects more interference of disease with function.
a
Comparability to prior tests is limited as patient’s home portable oxygen concentrator, used on prior tests, was unavailable thus the patient carried a portable oxygen tank. 6MWT values here are likely not fully reflective of patient’s ability.
To cite this abstract in AMA style:
Torok K, Rosser F, Rose-Felker K, Sood V, Kurland G, Olson A, Frost M, Vandergrift V, McIntyre S, Maslanka A, Schollaert-Fitch K, Barnum J, Szabolcs P. Autologous Stem Cell Transplantation with CD34-Selected Peripheral Blood Stem Cells in Patients with Treatment-Resistant Juvenile-Onset Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/autologous-stem-cell-transplantation-with-cd34-selected-peripheral-blood-stem-cells-in-patients-with-treatment-resistant-juvenile-onset-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/autologous-stem-cell-transplantation-with-cd34-selected-peripheral-blood-stem-cells-in-patients-with-treatment-resistant-juvenile-onset-systemic-sclerosis/