Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Several autoantibodies have been linked to various disease manifestations of systemic sclerosis (SSc). Anti-RNA polymerase 3 (ARA) and anti-topoisomerase-I (ATA) are both associated with diffuse cutaneous (dc)SSc. ARA is associated with rapidly progressive skin thickening, scleroderma renal crisis (SRC), malignancy, and gastric antral vascular ectasia (GAVE), while ATA is associated with severe, progressive interstitial lung disease (ILD). These autoantibody associations described in the literature originated from studies of heterogenous SSc populations. Whether these associations with ARA and ATA hold true in a cohort of patients with early dcSSc is unknown. The aim of this study was to examine the associations of ATA and ARA with SSc clinical manifestations in adults with early dcSSc.
Methods: The Prospective Registry of Early Systemic Sclerosis (PRESS) is a cohort of adults with early dcSSc with disease duration ≤2 years from onset of the first non-Raynaud symptom. All participants met ACR/EULAR 2013 classification criteria for SSc. Participants were recruited from 12 academic medical centers in the United States from April 2012 to July 2020. Data were captured at enrollment and every 6 months thereafter. We used the statistical tests outlined in the Table 1 footnote to compare differences between groups.
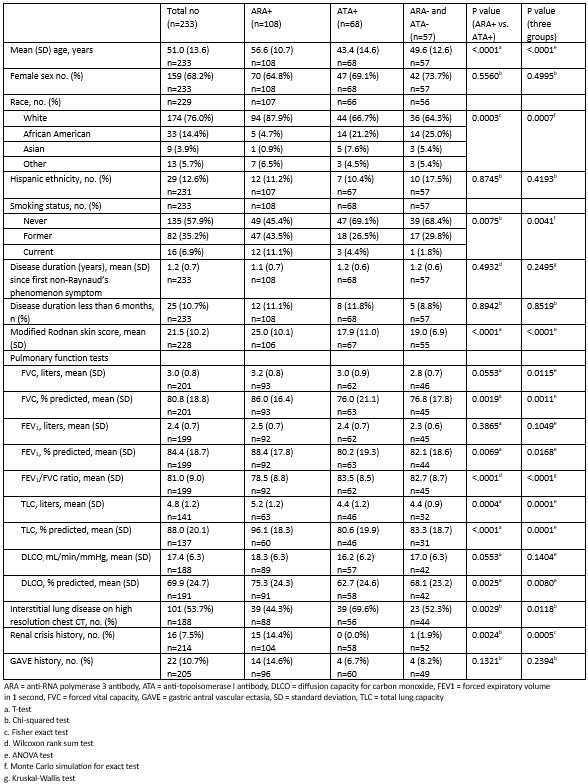
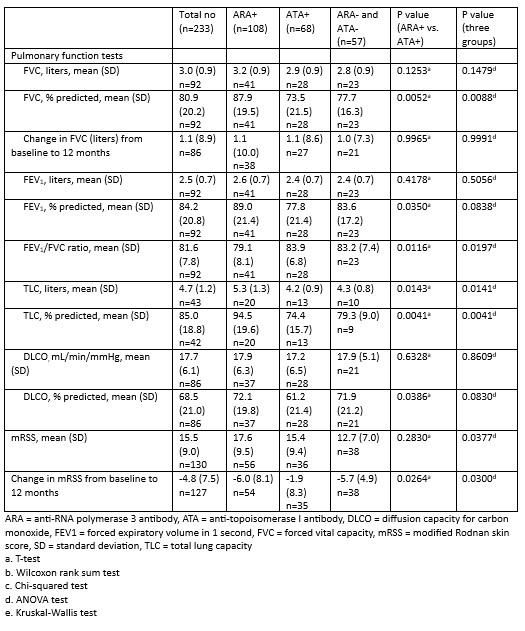
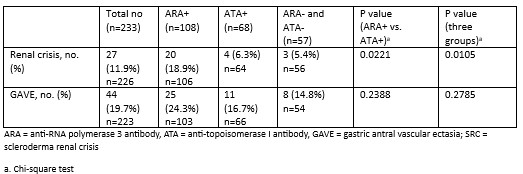
Results: 233 of the 303 patients enrolled in PRESS were included in this study. Those with missing autoantibody data (n = 66) and those positive for both ARA and ATA (n = 4) were excluded. 108 were ARA+, 68 were ATA+, and 57 were negative for both (“double negative”) (Table 1). The mean ± standard deviation (SD) age was 51 (13.6) years. Most were female (68.2%) and White (76%). The mean ± SD disease duration was 1.2 ± 0.7 years. At baseline, the forced vital capacity percent predicted (FVC%) was significantly lower in the ATA+ and double negative groups compared to the ARA+ group (76% vs 76.8% vs 86%, p = 0.0011). A significantly higher proportion of ATA+ patients had ILD on baseline high resolution computed tomography scan of the chest (HRCT) compared to ARA+ patients (69.6% vs 44.3%, p=0.0029). A greater proportion of ARA+ patients had a history of SRC at baseline compared to the ATA+ and double negative groups (14.4% vs 0% vs 1.9%, p = 0.0005). ARA+ patients had a higher mean ± SD modified Rodnan Skin Score (mRSS) at baseline than ATA+ and double negative patients (25.5 ± 10.2 vs 17.9 ± 11 vs 19 ± 6.9, p < 0.0001). At 12 months, there was no difference in the change in FVC between groups. There was a greater decline in mRSS in the ARA+ group compared to the ATA+ group at 12 months (p=0.0264; Table 2). Although a greater proportion of ARA+ patients developed GAVE, this difference was not significant (Table 3).
Conclusion: ATA+ patients had lower FVC% and a higher prevalence of ILD on baseline HRCT compared to ARA+ and double negative patients. ARA+ patients had a higher baseline mRSS and a greater proportion had SRC. The double negative group had lower PFT parameters and a higher prevalence of ILD compared to the ARA+ group. Organ manifestations of SSc were present early, often at baseline. This highlights the importance of early screening for major organ involvement in dcSSc.
To cite this abstract in AMA style:
Agarwal A, Hassan D, Huang S, Castelino F, Assassi S, Domsic R, Frech T, Gordon J, Hant F, Hinchcliff M, Shah A, Shanmugam V, Steen V, Khanna D, Bernstein E. Autoantibody Associations with Disease Manifestations in Patients with Early Diffuse Cutaneous Systemic Sclerosis: The Prospective Registry of Early Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/autoantibody-associations-with-disease-manifestations-in-patients-with-early-diffuse-cutaneous-systemic-sclerosis-the-prospective-registry-of-early-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/autoantibody-associations-with-disease-manifestations-in-patients-with-early-diffuse-cutaneous-systemic-sclerosis-the-prospective-registry-of-early-systemic-sclerosis/