Session Information
Session Type: Abstract Session
Session Time: 10:30AM-10:45AM
Background/Purpose: The release of neutrophil extracellular traps (NETs) by hyperactive neutrophils is recognized to play an important role in the thromboinflammatory milieu inherent to severe presentations of COVID-19. At the same time, a variety of functional autoantibodies have been observed in individuals with severe COVID-19 where they likely contribute to immunopathology. Work by our group and others has found that many hospitalized COVID-19 patients develop antiphospholipid antibodies similar to those found in antiphospholipid syndrome (APS). Patients with APS (as well as lupus and ANCA-associated vasculitis) have also been reported to develop autoantibodies targeting NETs, which impair NET clearance and activate complement. Here, we aimed to determine the extent to which autoantibodies might target NETs in COVID-19 and, if detected, to elucidate their clinical associations and potential functions.
Methods: NETs were solubilized and coated onto ELISA plates. We then measured IgG and IgM global anti-NET activity in 328 individuals hospitalized with COVID-19 alongside 48 healthy controls. The ability of COVID sera and purified anti-NET antibodies to protect NETs from degradation was determined. We assessed complement deposition on NETs by immunofluorescence microscopy.
Results: We found elevated levels of anti-NET IgG and IgM in patients with COVID-19 as compared with healthy controls (p< 0.0001 for both IgG and IgM) (Figure 1A-B). Using a cutoff of 2 standard deviations above the control mean, 27% of patients were positive for anti-NET IgG and 60% for anti-NET IgM. There was a strong interrelationship between anti-NET IgG and anti-NET IgM (r=0.4, p< 0.0001). High anti-NET antibody levels associated with circulating markers of NETs. Specifically, anti-NET IgG and IgM levels were positively correlated with myeloperoxidase-DNA complexes (IgG r=0.31, p< 0.0001; IgM r=0.41, p< 0.0001) and calprotectin (IgG r=0.33, p< 0.0001; IgM r=0.34, p< 0.0001). Clinically, anti-NET antibodies tracked with impaired oxygenation efficiency defined as SpO2/FiO2 ratio (IgG r=-0.17, p=0.018; IgM r=-0.30, p< 0.0001) and elevated levels of circulating D-dimer (IgG r=0.33, p< 0.0001; IgM r=0.19, p=0.0012). Furthermore, patients who required mechanical ventilation had higher levels of anti-NET antibodies than those who did not require oxygen supplementation (IgG p=0.0013; IgM p< 0.0001). Mechanistically, levels of anti-NET IgG in particular demonstrated a negative correlation with the NET degradation potential of COVID sera (Figure 1C), while purified IgG from high anti-NET sera impaired the ability of DNases in healthy serum to degrade NETs (Figure 1D). As compared with low anti-NET COVID sera, high anti-NET sera also more efficiently deposited complement C3d on NETs (Figure 2).
Conclusion: In summary, these data reveal high levels of anti-NET antibodies in many individuals hospitalized with COVID-19, where they track with NETs themselves and also disease severity. It is likely that these antibodies impair NET clearance and potentiate complement activation, which may together conspire to potentiate SARS-CoV-2-mediated thromboinflammation.
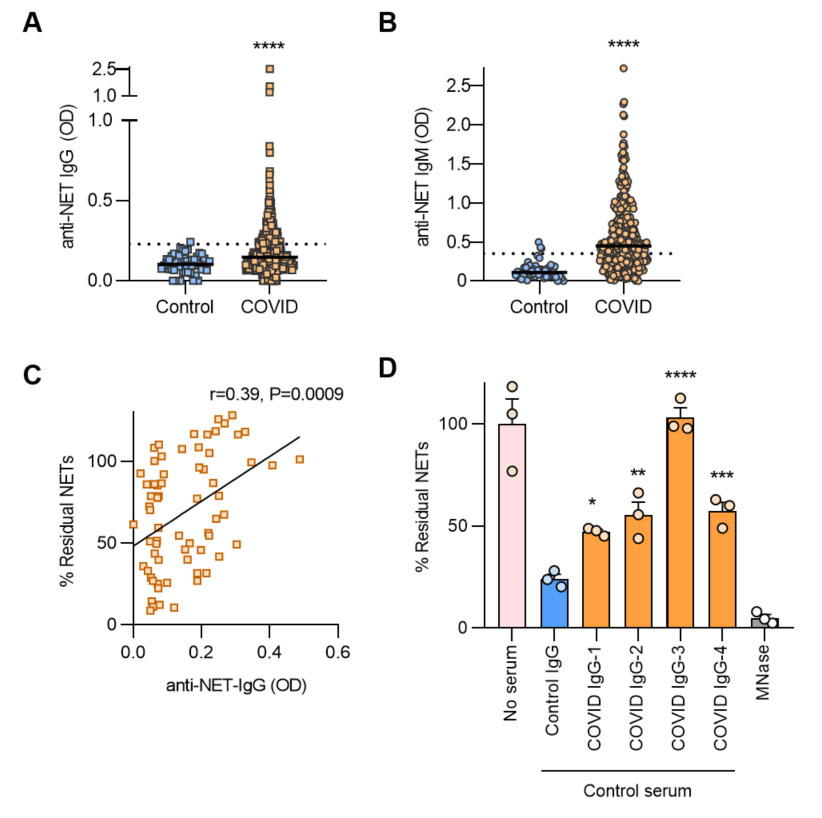 Figure 1. A-B, Anti-NET IgG/M levels were compared by Mann-Whitney test; ****p < 0.0001. Dotted lines indicate positive threshold set at 2 standard deviations above the control mean. C, Freshly-induced NETs were incubated with sera from COVID_19 patients. After 90 minutes, percent residual NETs were determined. Correlation with anti-NET IgG was determined by Spearman’s method. D, Freshly-induced NETs were incubated with control serum supplemented with either purified IgG from COVID patients or controls, and percent residual NETs were determined after 90 minutes. COVID IgG was compared to control by one-way ANOVA; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Figure 1. A-B, Anti-NET IgG/M levels were compared by Mann-Whitney test; ****p < 0.0001. Dotted lines indicate positive threshold set at 2 standard deviations above the control mean. C, Freshly-induced NETs were incubated with sera from COVID_19 patients. After 90 minutes, percent residual NETs were determined. Correlation with anti-NET IgG was determined by Spearman’s method. D, Freshly-induced NETs were incubated with control serum supplemented with either purified IgG from COVID patients or controls, and percent residual NETs were determined after 90 minutes. COVID IgG was compared to control by one-way ANOVA; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
 Figure 2. Control neutrophils were stimulated with PMA to generate NETs. After fixation with paraformaldehyde, NETs were incubated with sera from patients with high (top panels) or low (bottom panels) anti-NET IgG; scale bars=100 microns.
Figure 2. Control neutrophils were stimulated with PMA to generate NETs. After fixation with paraformaldehyde, NETs were incubated with sera from patients with high (top panels) or low (bottom panels) anti-NET IgG; scale bars=100 microns.
To cite this abstract in AMA style:
Zuo Y, Yalavarthi S, Navaz S, Hoy C, Harbaugh A, Gockman K, Zuo M, Madison J, Shi H, Kanthi Y, Knight J. Autoantibodies Stabilize Neutrophil Extracellular Traps in COVID-19 [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/autoantibodies-stabilize-neutrophil-extracellular-traps-in-covid-19/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/autoantibodies-stabilize-neutrophil-extracellular-traps-in-covid-19/
