Session Information
Date: Monday, November 14, 2022
Title: Systemic Sclerosis and Related Disorders – Clinical Poster III
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: We sought to describe the autoantibody profile of SSc patients with early disease and examine the clinical, laboratory and prognostic features associated with these antibody subsets.
Methods: Study participants were from the Collaborative National Quality and Efficacy Registry (CONQUER), a multicenter US-based registry of SSc patients within 5 years (median 2.2-2.8 years) of their first non-Raynaud’s symptom. As of April 25, 2022, 533 patients were enrolled and had autoantibody testing as part of their clinical care. All subjects met 2013 ACR/ European League Against Rheumatism criteria for SSc. Baseline and most abnormal characteristics during follow-up were compared by antibody status.
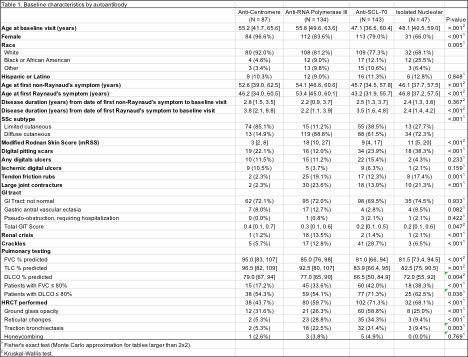
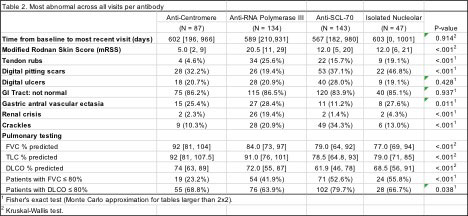
Results: Of the 533 patients evaluated, the vast majority had a positive ANA screen; only 3.7% were ANA negative. 87 patients (16.3%) had ACA, 134 (25.1%) anti-RNA polymerase III (RNA pol III), 143 (26.8%) Scl-70, and 47 (8.8%) had an isolated nucleolar antibody pattern without other subserology. There were 5% of patients who had other SSc-associated subserologies, including 14 with anti-Pm/Scl, 3 anti-U3-RNP (also nucleolar ANA patterns) and 10 with U1-RNP. Almost 10% were ANA positive but did not have any SSc subserology, while 4.3%% had 2 or more subserologies. ACA patients were likely to be female Caucasians with limited cutaneous disease. They had the lowest modified Rodnan Skin Scores (mRSS) and highest SSc-GI tract (GIT) scores across groups. RNA pol III was strongly associated with diffuse cutaneous disease, having the most severe skin involvement of the groups by mRSS score and frequency of joint contractures and tendon friction rubs. Patients with RNA pol III had the highest rate of scleroderma renal crisis. Scl-70 patients were youngest at symptom onset; additionally, they had the most severe lung disease on pulmonary function tests (PFTs) and high-resolution CT imaging. Patients with an isolated nucleolar antibody were demographically and clinically most similar to Scl-70 patients; these groups had high proportions of African-American and male patients as well as more severe PFTs than other antibody groups. In the 1.6 years of follow up, the lowest forced vital capacity (FVC) measurements were seen in Scl-70 and isolated nucleolar antibody patients with median FVCs of 79% and 77% predicted, respectively (p< 0.001) (Table 2).
Conclusion: Even in early disease, ACA, RNA pol III and Scl-70 have the typical clinical associations that have been established with these antibodies. Patients with an isolated nucleolar antibody pattern without other SSc subserology should be followed closely for interstitial lung disease.
To cite this abstract in AMA style:
Bosso A, Assassi S, Frech T, Gordon J, Bernstein E, Richardson C, Sandorfi N, Hummers L, Shah A, Khanna D, Chung L, Castelino F, Hant F, Shanmugam V, VanBuren J, Larkin A, Evnin L, Steen V. Autoantibodies in Patients with Early Systemic Sclerosis in the Collaborative National Quality and Efficacy Registry [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/autoantibodies-in-patients-with-early-systemic-sclerosis-in-the-collaborative-national-quality-and-efficacy-registry/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/autoantibodies-in-patients-with-early-systemic-sclerosis-in-the-collaborative-national-quality-and-efficacy-registry/