Session Information
Date: Monday, November 8, 2021
Title: Abstracts: SLE – Treatment: New Agents, Old Agents (1458–1463)
Session Type: Abstract Session
Session Time: 3:45PM-4:00PM
Background/Purpose: The Lupus Low Disease Activity State (LLDAS), a treat-to-target (T2T) endpoint for SLE, is prospectively validated as protective from flares and damage accrual.1 LLDAS attainment is therefore an SLE treatment goal, and was achieved more frequently with active treatment than placebo in 2 SLE phase 2 trials.2,3 In the TULIP-2 (NCT02446899) phase 3 trial, efficacy of anifrolumab, a type I IFN receptor mAb, was demonstrated using the BILAG–based Combined Lupus Assessment (BICLA) response, and these findings were supported in TULIP-1 (NCT02446912).4,5 We investigated if anifrolumab treatment was associated with increased LLDAS attainment in pooled TULIP data.
Methods: TULIP-1/2 were randomized, placebo-controlled, 52-week trials of IV anifrolumab (Q4W, 48 weeks) in eligible patients meeting ACR 1997 criteria for SLE with moderate to severe SLE despite standard therapy. Pooled data for the anifrolumab 300 mg and placebo groups were analyzed by time point for LLDAS attainment (defined as all of the following: SLEDAI-2K <4 without major organ activity, no new disease activity, Physician’s Global Assessment [0–3] <1, prednisone or equivalent <7.5 mg/day, and well-tolerated standard immunosuppressant dosing6). Time to first LLDAS attainment was compared between treatment groups using Cox regression, cumulative time in LLDAS was compared using a Cochran–Mantel–Haenszel approach, and responses were compared using logistic regression. All P-values are nominal.
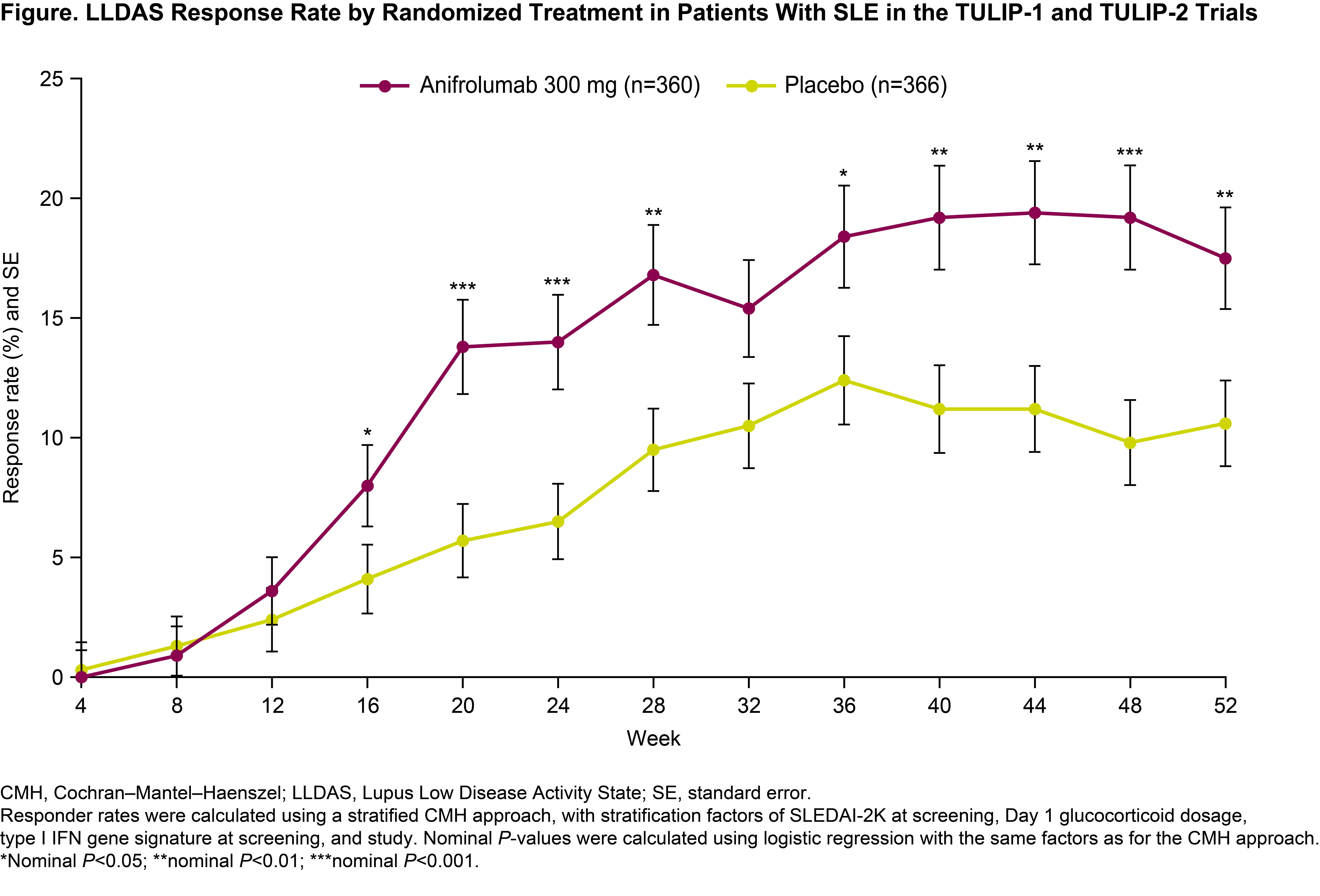
Results: In an analysis agnostic to treatment group, 114 (13.9%) patients attained LLDAS at Week 52. Of these patients, 102 (89.5%) were also BICLA responders (analysis included the TULIP-1 anifrolumab 150 mg group). Among the 318 BICLA responders at Week 52, 102 (32.0%) attained LLDAS. LLDAS attainment increased across the 52-week trial and was attained earlier with anifrolumab 300 mg than placebo (Figure) (time to first LLDAS, HR 1.76, 95% CI 1.35–2.30, P< 0.001). At Week 52, 17.5% of anifrolumab-treated and 10.6% of placebo patients were in LLDAS (OR 1.8, 95% CI 1.2–2.8, P=0.008). More cumulative time (P< 0.001) and percentage of time (P < 0.001) was spent in LLDAS by patients receiving anifrolumab than placebo, and cumulative time in LLDAS thresholds of ≥20% (OR 1.8, 95% CI 1.2–2.7, P=0.004) and ≥50% (OR 1.9, 95% CI 1.0–3.4, P=0.035) also favored anifrolumab. Anifrolumab-treated patients were more likely to be in sustained LLDAS for ≥3 consecutive visits (18.6% vs 12.5%, OR 1.6, 95% CI 1.1–2.4, P=0.024), ≥5 consecutive visits (11.2% vs 7.7%, OR 1.5, 95% CI 0.9–2.6, P=0.106), or ≥7 consecutive visits (6.8% vs 2.7%, OR 2.6, 95% CI 1.2–5.6, P=0.014).
Conclusion: LLDAS is an attainable T2T endpoint in SLE clinical trials. LLDAS is more stringent than BICLA, but LLDAS attainment is highly coincident with BICLA response. Anifrolumab treatment was associated with earlier, more frequent, more prolonged, and more sustained LLDAS attainment.
References:
1. Golder V. Lancet Rheumatol 2019;1:e95–102.
2. Wallace D. Lancet 2018;392:222–31.
3. Morand E. Ann Rheum Dis 2018;77:706–13.
4. Morand EF. New Engl J Med 2020;382:211–21.
5. Furie R. Lancet Rheumatol 2019;1:e208–19.
6. Franklyn K. Ann Rheum Dis 2016;75:1615–21.
To cite this abstract in AMA style:
Morand E, Abreu G, Furie R, Tummala R. Attainment of the Lupus Low Disease Activity State in Response to Anifrolumab in 2 Phase 3 Trials [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/attainment-of-the-lupus-low-disease-activity-state-in-response-to-anifrolumab-in-2-phase-3-trials/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/attainment-of-the-lupus-low-disease-activity-state-in-response-to-anifrolumab-in-2-phase-3-trials/