Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Cholesterol efflux capacity CEC measures the ability of high-density lipoprotein (HDL) to remove cholesterol from arterial wall macrophages upon interaction with membrane transporter proteins and reduce the lipid content of atherosclerotic plaques. CEC inversely associated with cardiovascular risk in general patients independently of HDL levels. CEC though the ATP-binding-cassette G1 (ABCG1) membrane transporter is impaired in RA, yet, its relationship to atherosclerosis and cardiovascular risk is unknown. We here evaluated associations of ABCG1-mediated CEC with coronary atherosclerosis burden, plaque progression and incident cardiovascular event risk in RA.
Methods: Coronary atherosclerosis was evaluated with computed tomography angiography in 140 patients without cardiovascular disease and reassessed in 99 after 83.6±4.0 months. ABCG1-mediated CEC was measured in Chinese hamster ovary cells, not transfected or transfected with ABCG1 gene, as percentage of effluxed cholesterol over total intracellular cholesterol content. Logistic and negative binomial regression tested associations of ABCG1 with binary and count plaque outcomes respectively. Cox regression evaluated the association of ABCG1 with long-term cardiovascular event risk.
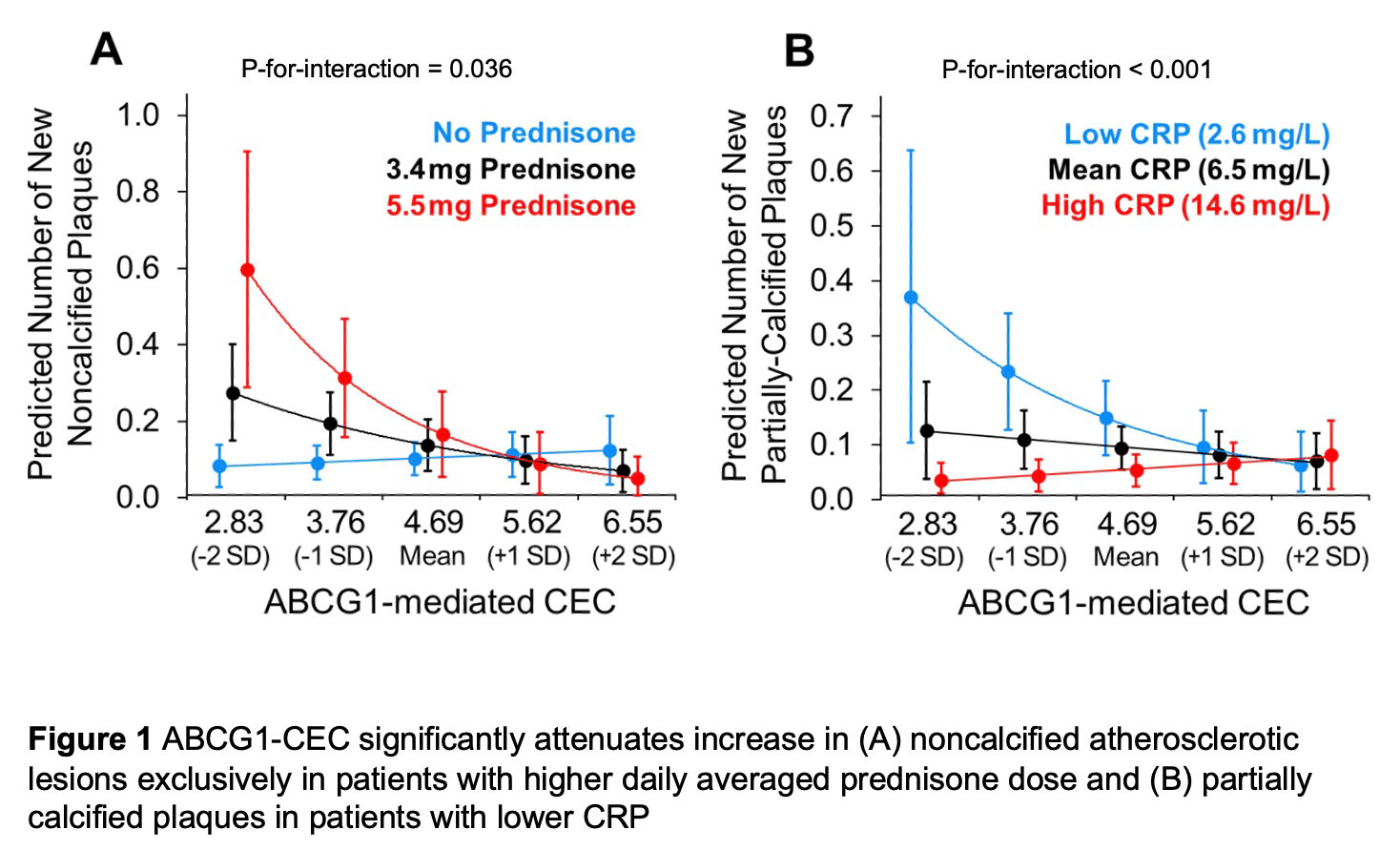
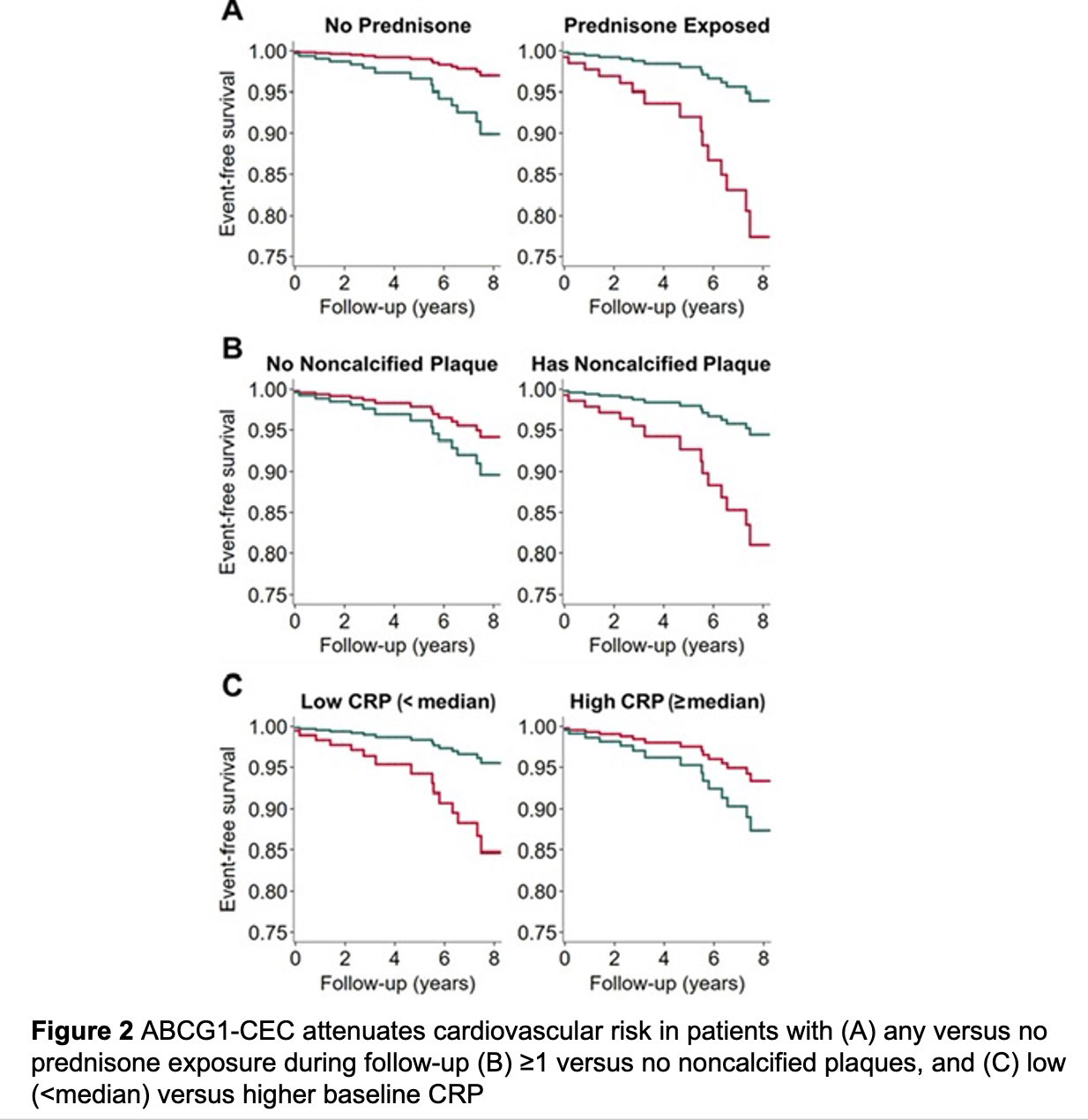
Results: Mean (standard deviation [SD]) of ABCG1 was 4.71 (0.92)%. At baseline, ABCG1 inversely associated with likelihood of extensive atherosclerosis (≥5 plaques) (odds ratio 0.50 [95% CI 0.28-0.88]) and numbers of partially-calcified (rate ratio [RR] 0.71 [95% CI 0.53-0.94]) and low-attenuation plaques (RR 0.63 [95% CI 0.43-0.91]) independently of ASCVD score and statin use. There were no main effects of ABCG1-CEC in adjusted plaque progression models. However, higher ABCG1-CEC predicted decreased noncalcified and calcified plaque progression exclusively in patients with higher time-weighted mean prednisone dose (Figure 1A). Furthermore, ABCG1-CEC inversely associated with progression of partially-calcified plaque at lower time-averaged CRP levels (Figure 1B). ABCG1-CEC did not have a main effect on cardiovascular event risk after adjusting for ASCVD score. However, higher ABCG1 associated with lower risk in patients with any versus no prednisone exposure during follow-up, ≥1 versus no noncalcified plaques, and low (< median) versus higher baseline CRP after adjustments including for ASCVD score (p-for-interaction=0.008, 0.021 and 0.033 respectively, Figure 2)
Conclusion: ABCG1-mediated CEC inversely associated with coronary plaque burden and number of high-risk plaques at baseline. It further predicted decreased plaque progression conditionally on cumulative inflammation and prednisone dose. Notably, ABCG1-mediated CEC inversely associated with cardiovascular risk specifically in prednisone users, patients with noncalcified plaques at baseline and those with lower inflammation.
To cite this abstract in AMA style:
Karpouzas G, Papotti b, Ormseth S, Palumbo M, Hernandez e, Adorni M, Zimetti F, Budoff M, Ronda N. ATP-Binding Cassette G1 Membrane Transporter-mediated Cholesterol Efflux Capacity Influences Coronary Atherosclerosis and Cardiovascular Risk in RA [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/atp-binding-cassette-g1-membrane-transporter-mediated-cholesterol-efflux-capacity-influences-coronary-atherosclerosis-and-cardiovascular-risk-in-ra/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/atp-binding-cassette-g1-membrane-transporter-mediated-cholesterol-efflux-capacity-influences-coronary-atherosclerosis-and-cardiovascular-risk-in-ra/