Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: While the incidence of severe acute COVID-19 has decreased, post-acute sequelae of COVID-19 characterized by prolonged symptoms, or ‘long COVID,’ is common and associated with lower quality of life and morbidity. Long COVID may be in part driven by systemic inflammation and immune dysregulation. Patients with systemic autoimmune rheumatic diseases (SARDs) may be at risk for long COVID due to underlying altered immunity, immunosuppressive drug use, and propensity for systemic inflammation. Thus, identifying inflammatory biomarkers for long COVID may be helpful to identify biologic pathways and develop diagnostic tests.
Methods: We investigated biomarkers of inflammation and long COVID using RheumCARD, a prospective study of people with prevalent SARDs with COVID-19 from a large healthcare system. Participants are recruited ≥28 days following onset of acute COVID-19 to answer surveys and provide blood samples (March 2021 to July 2023). Circulating cytokines were measured in serum using the Olink Target 48 cytokine panel. If patients were on a DMARD targeted to a cytokine (e.g., TNF), that result was excluded. In the primary analysis, long COVID was defined as symptoms persisting for ≥28 days, comparing those with vs. without long COVID. We performed subgroup analyses among those with inflammatory arthritis, pre-Omicron variants, Omicron variants, remission/low disease activity, moderate/high disease activity as well as a more stringent definition of long COVID (≥90 days of persistent symptoms). We compared cytokine levels between those with vs. without long COVID using linear regression.
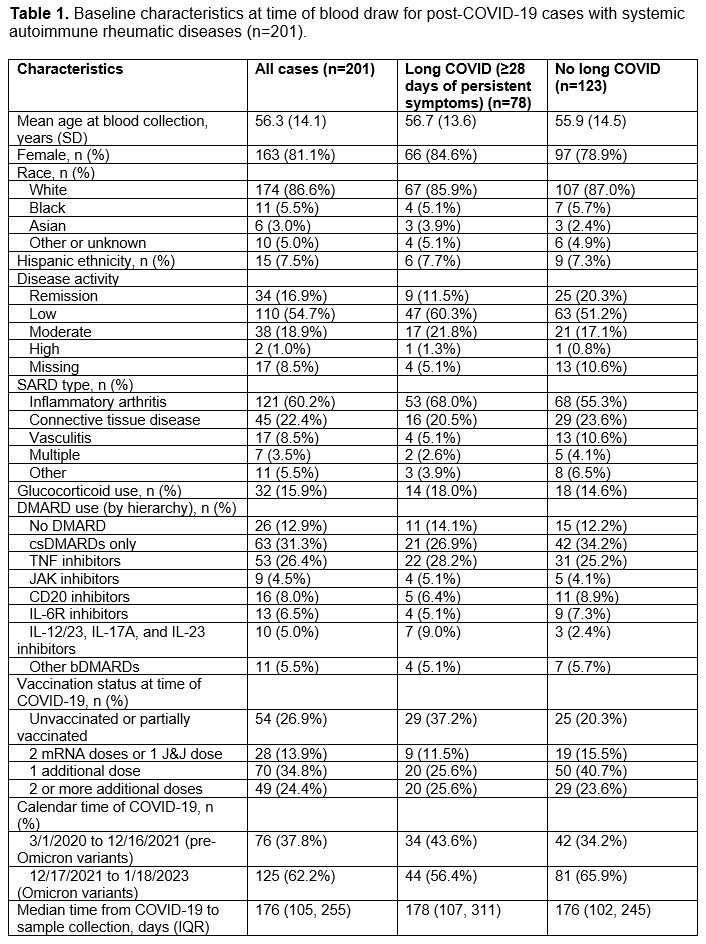
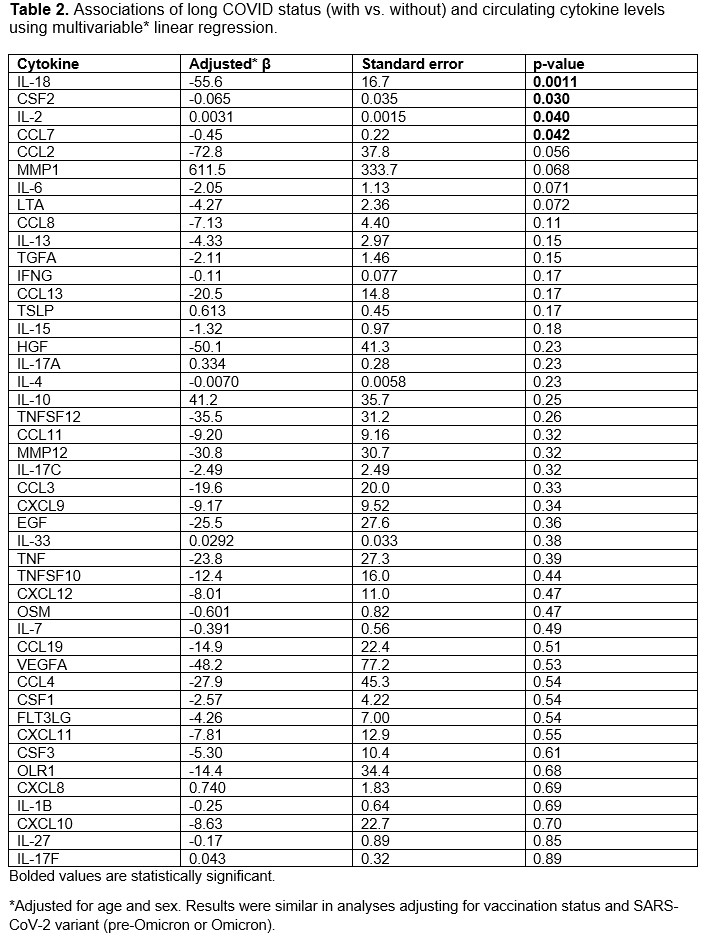
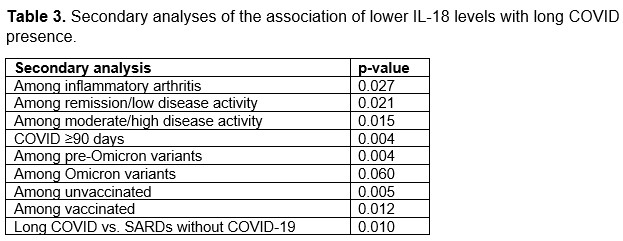
Results: We analyzed a total of 201 participants (mean age 56.3 years, 81% female). The most common SARD type among cases was inflammatory arthritis (60%), followed by connective tissue disease (22%, Table 1). IL-18 levels were lower among those with long COVID (199 units) vs. without long COVID (221 units; p=0.001). In the multivariable analysis, long COVID cases had lower IL-18 levels than those without long COVID (β -55 units, SE 17, p=0.0011, Table 2). There were also associations of CSF2, IL2, and CCL6 with long COVID. IL-18 levels were consistently lower among those with long COVID in all additional analyses (Table 3): inflammatory arthritis (p=0.021), remission/low disease activity (p=0.02), moderate/high disease activity (p=0.015), pre-Omicron variants (0.004), Omicron variants (p=0.06), long COVID defined as ≥90 days of persistent symptoms (p=0.004), and vs. comparators (p=0.011).
Conclusion: In this prospective study performed among SARDs after COVID-19, lower IL-18 levels were associated with higher risk of long COVID. This finding was robust across all analyses examined and not explained by vaccination or viral variants. IL-18 induces cell-mediated immunity following infection, implicating a blunted immune response, rather than exuberant hyperinflammation, as a potential mechanism for long COVID among patients with SARDs.
To cite this abstract in AMA style:
Sparks J, Wang X, Lee P, Brodeur K, Lin M, Patel N, Kawano Y, Schiff A, King A, Hanberg J, Srivatsan S, Kowalski E, Johnson C, Vanni K, Williams Z, Qian G, Bolden C, Mueller K, Bade K, Saavedra A, Venkat R, Wallace Z. Associations of Circulating Inflammatory Cytokines with Long COVID Among Patients with Systemic Autoimmune Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/associations-of-circulating-inflammatory-cytokines-with-long-covid-among-patients-with-systemic-autoimmune-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/associations-of-circulating-inflammatory-cytokines-with-long-covid-among-patients-with-systemic-autoimmune-rheumatic-diseases/