Session Information
Date: Sunday, November 7, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster II: Manifestations (0855–0896)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Systemic lupus erythematosus (SLE) occurs predominantly in women and Black women have disproportionately poorer health outcomes across the trajectory of their disease compared to women of other race/ethnicities. Specifically, Black women with SLE report higher symptom burden with pervasive impacts on quality of life. Fatigue is highly prevalent in SLE patients, and is a major driver of negative disease perception and patient difficulty with steroid therapy discontinuation. However, the underlying biochemical mechanisms of fatigue in Black women with SLE are largely unknown. We conducted untargeted metabolomic plasma profiling in Black females with SLE and Black female non-SLE controls to identify metabolites and metabolic pathways associated with SLE. We then identified which of these SLE metabolites and pathway predictors were correlated with patient-reported symptoms of fatigue.
Methods: Blood samples were collected from 23 Black female patients with diagnosis of SLE during a routine outpatient rheumatology visit and from 21 Black female non-SLE controls. SLE clinical data were obtained via EMR review and all subjects completed identical, reliable and valid measures of fatigue (Multidimensional Fatigue Inventory [MFI]) and PROMIS symptom measures. A US-based commercial metabolomics analysis company conducted untargeted metabolomics on the 44 plasma samples using ultrahigh performance liquid chromatography/tandem mass spectrometry along with metabolite identification and quantification to examine differences between SLE/non-SLE groups. Metabolites and symptom data were correlated using Kendall’s Tau with a two tailed alpha of p < .05.
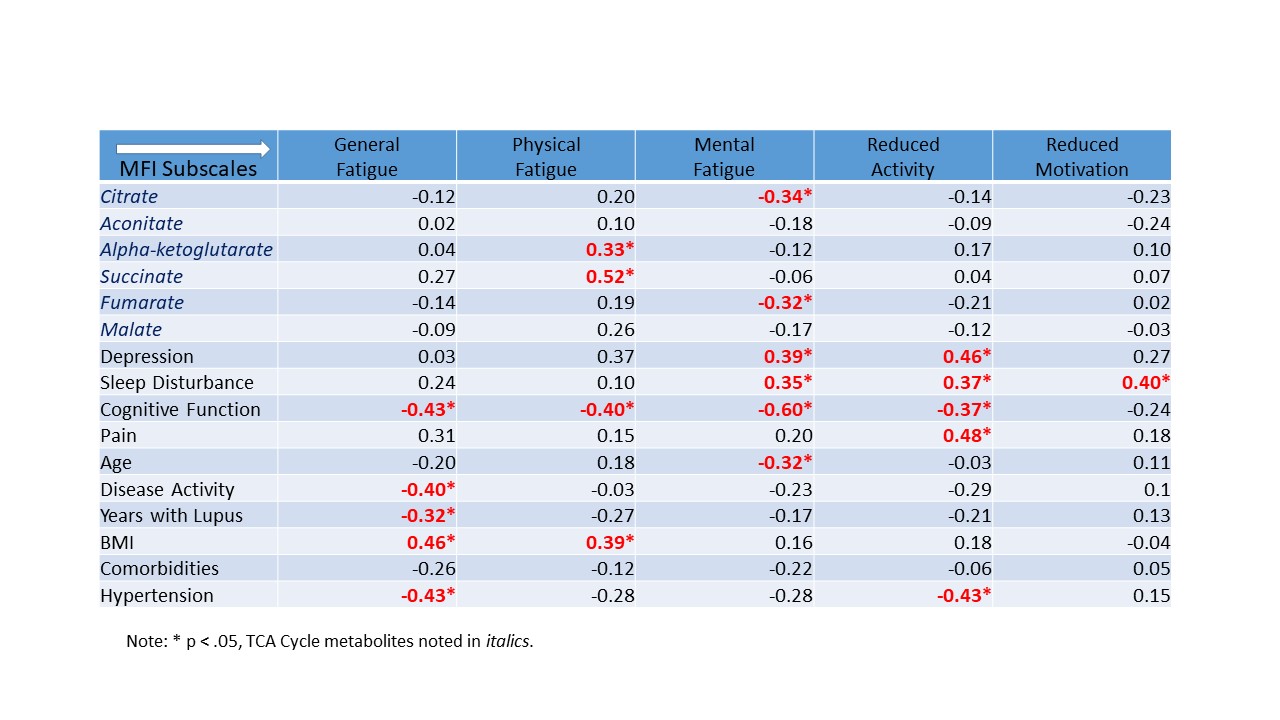
Results: All SLE subjects met 2019 EULAR/ACR diagnostic criteria. SLE subjects were significantly younger (42.5 years ± 12.2 vs. 63.2 years ± 6.4), had a lower BMI (30.3 ± 9.4 vs. 34.9 ± 4.1), and greater co-morbidities (2.3 ± 1.3 vs. 1.1 ± 1.3) than non-SLE controls. Welch’s two sample t-tests revealed 290 biochemicals that were significantly different (p < .05) between SLE and non-SLE groups; with Random Forest analysis yielding a predictive accuracy of 91% in differing between the two groups. While significant metabolic differences between groups were noted in biochemicals within pathways associated with glycolysis, the TCA cycle, fatty acid metabolism, branched chain amino acids, sterols, and heme catabolism, the metabolites that associated with fatigue were within the TCA cycle, also called the Kreb’s Cycle, and responsible for energy production (See table). Increased MFI physical fatigue was significantly correlated with alpha-ketoglutarate, succinate, lower cognitive function, and greater BMI. Increased MFI mental fatigue was significantly associated with fumarate, depression, sleep disturbance, lower cognitive function, and increased age.
Conclusion: Metabolites from the TCA cycle, identified through untargeted metabolomics, were significantly associated with physical and mental fatigue phenotypes in Black females with SLE. Validating these findings in a larger sample would inform potential biomarkers for fatigue as well as targets for intervention.
To cite this abstract in AMA style:
Kimble L, Khosroshahi A, Brewster G, Carlson N, Eldridge R, Corwin E. Associations Between Tricarboxylic Acid Cycle Plasma Metabolites and Fatigue Phenotypes in Black Females with Systemic Lupus Erythematosus: An Untargeted Metabolomics Analysis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/associations-between-tricarboxylic-acid-cycle-plasma-metabolites-and-fatigue-phenotypes-in-black-females-with-systemic-lupus-erythematosus-an-untargeted-metabolomics-analysis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/associations-between-tricarboxylic-acid-cycle-plasma-metabolites-and-fatigue-phenotypes-in-black-females-with-systemic-lupus-erythematosus-an-untargeted-metabolomics-analysis/