Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Drug development in SLE is informed by US Food and Drug Administration recommendations for study design, enrollment, and efficacy endpoints. However, persistent challenges to conducting high-quality randomized clinical trials (RCTs) have been noted, including variation in endpoint assessment, predominantly urban enrollment, and underrepresentation of minoritized populations. RCT quality contributes to the perceived strength of research findings, yet little is known about heterogeneity in trial design, including potential associations between representation and the quality of RCTs in SLE.
Methods: We identified RCTs of small molecules and biologics for SLE registered on ClinicalTrials.gov and published in peer-reviewed journals as of January 2025. Eligible RCTs evaluated a primary efficacy endpoint and included 1+ US enrollment site. US enrollment sites for RCTs were classified using the National Center for Health Statistics Urban-Rural Classification Scheme; medically underserved areas were identified. For each RCT, enrollment by sex, race, and ethnicity was identified from publications and compared to the demographics of US Centers for Disease Control and Prevention SLE registries using participation-to-prevalence ratios (PPRs). PPRs greater than 0.8 indicated adequate representation. RCT quality was assessed using the Cochrane Risk of Bias 2 tool. Associations between RCT enrollment and quality were evaluated using Fisher’s exact tests, with significance set at .05.
Results: There were publications for 54 RCTs and 1 pooled analysis of 2 RCTs. Median enrollment among 55 unique RCT reports was 298 (interquartile range, 130-449) (Table 1). Half of RCTs enrolled in at least one small metropolitan or non-metropolitan area in the US; however, most US sites (1392/1845, 75%) were in large metropolitan areas. Representation across RCTs was adequate for female (median PPR 1.0), Hispanic (median PPR 1.3), Asian (median PPR 2.1) and White (median PPR 1.0) participants, while American Indian/Alaska Native (AIAN, median PPR 0.7) and Black (median PPR 0.4) participants were underrepresented. Enrollment was similar regardless of whether therapeutics are approved for use in SLE. Among 55 RCTs, there were similar numbers with low risk (19, 34%), some concerns (19, 34%), or high risk (17, 30%) of bias (Table 2). There were no significant differences in RCT quality by geographic enrollment features. A significantly higher proportion of RCTs at high risk of bias was observed among those inadequately enrolling AIAN (P=.04) or White (P=.02) participants.
Conclusion: This study suggests that although numerous therapies have been investigated for use in SLE, US enrollment remains limited outside of large metropolitan areas, and enrollment overall underrepresents minoritized populations at risk of severe disease. Although RCT quality was not associated with geographic enrollment features, high risk of bias was less frequently observed among RCTs with adequate enrollment of AIAN and White participants. Variation in RCT quality is notable and may limit the generalizability of findings to real-world patients, contributing to uneven update and lack of clarity over the optimal use of promising therapies.
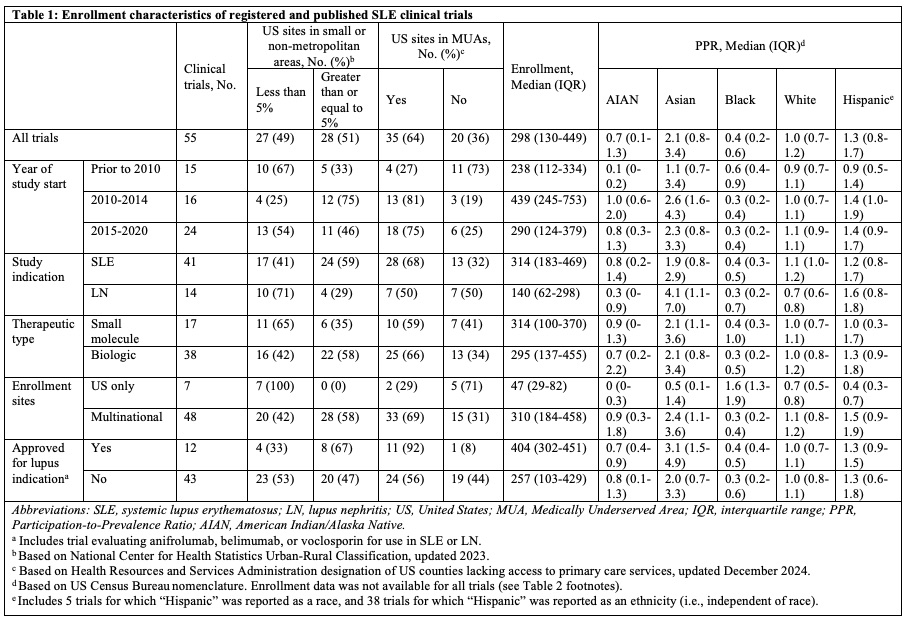 Table 1: Enrollment characteristics of registered and published SLE clinical trials
Table 1: Enrollment characteristics of registered and published SLE clinical trials
.jpg) Table 2: Cochrane Risk of Bias 2 analyses for SLE clinical trials
Table 2: Cochrane Risk of Bias 2 analyses for SLE clinical trials
To cite this abstract in AMA style:
Skydel J, Leland A, Ramachandran R, Koumpouras F, Wallach J. Associations between Representation and Quality in Clinical Trials for Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/associations-between-representation-and-quality-in-clinical-trials-for-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/associations-between-representation-and-quality-in-clinical-trials-for-systemic-lupus-erythematosus/
