Session Information
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Among patients with ANCA-associated vasculitis (AAV), several exhibit sinonasal involvement, especially in granulomatosis with polyangiitis (GPA) and eosinophilic granulomatosis with polyangiitis (EGPA). A recent study suggested that programmed death ligand 1 (PD-L1) expression in neutrophils is associated with dysfunction of NET degradation. Another study revealed that neutrophils from AAV patients highly expressed CD47 (the “don’t eat me” signal) and escaped from efferocytosis by macrophages expressing SIRPa, a ligand of CD47. Although several studies have shed light on the host microbiome, the connections between the nasal microbiome and autoimmune diseases remain frontier. We aimed to elucidate the association between the nasal microbiome and AAV (GPA, EGPA, and microscopic polyangiitis [MPA]) using next-generation sequencing and assessed correlations between these taxa and immune checkpoint molecules (PD-L1, CD47, and SIRPa).
Methods: This is a cross-sectional study. The nasal microbiome was compared among GPA, EGPA, and MPA. All patients with AAV were diagnosed based on Watts’ algorithm. Nasal samples were collected through oropharyngeal swabbing and analyzed by sequencing the bacterial 16S rDNA gene (V3-V4 region). Amplicon sequence variants (ASVs) were assigned in QIIME2 (2023.2) using the Greengenes 13_8. Bacterial taxa were assessed in the following analysis if their median abundance was >0% (relatively rare taxa was excluded). In each patient, monocytes, normal-density granulocytes (NDG) and low-density granulocytes (LDG) were isolated from peripheral blood using separation liquid. PD-L1, CD47, and SIRPa expression were assessed by flowcytometry.
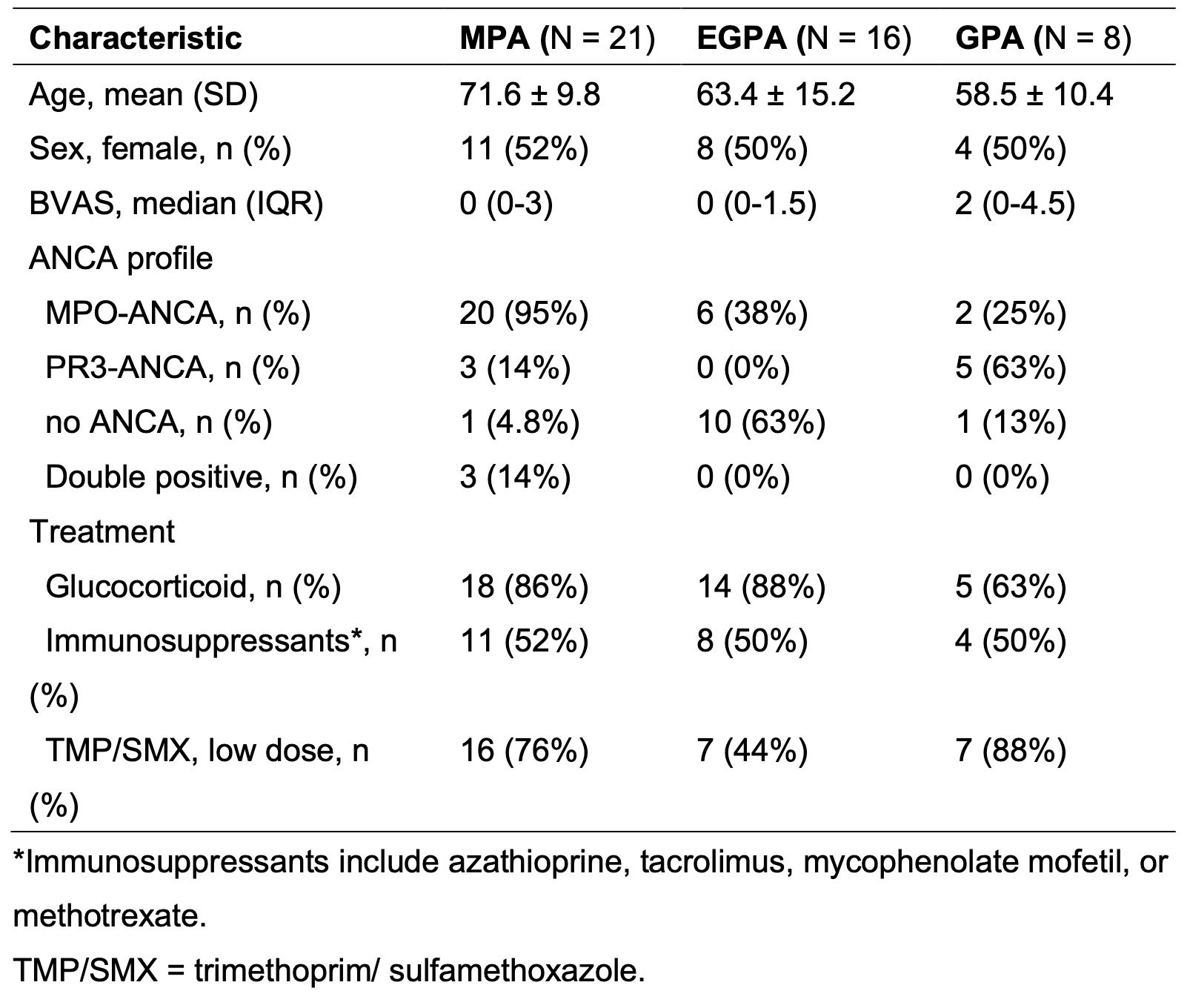
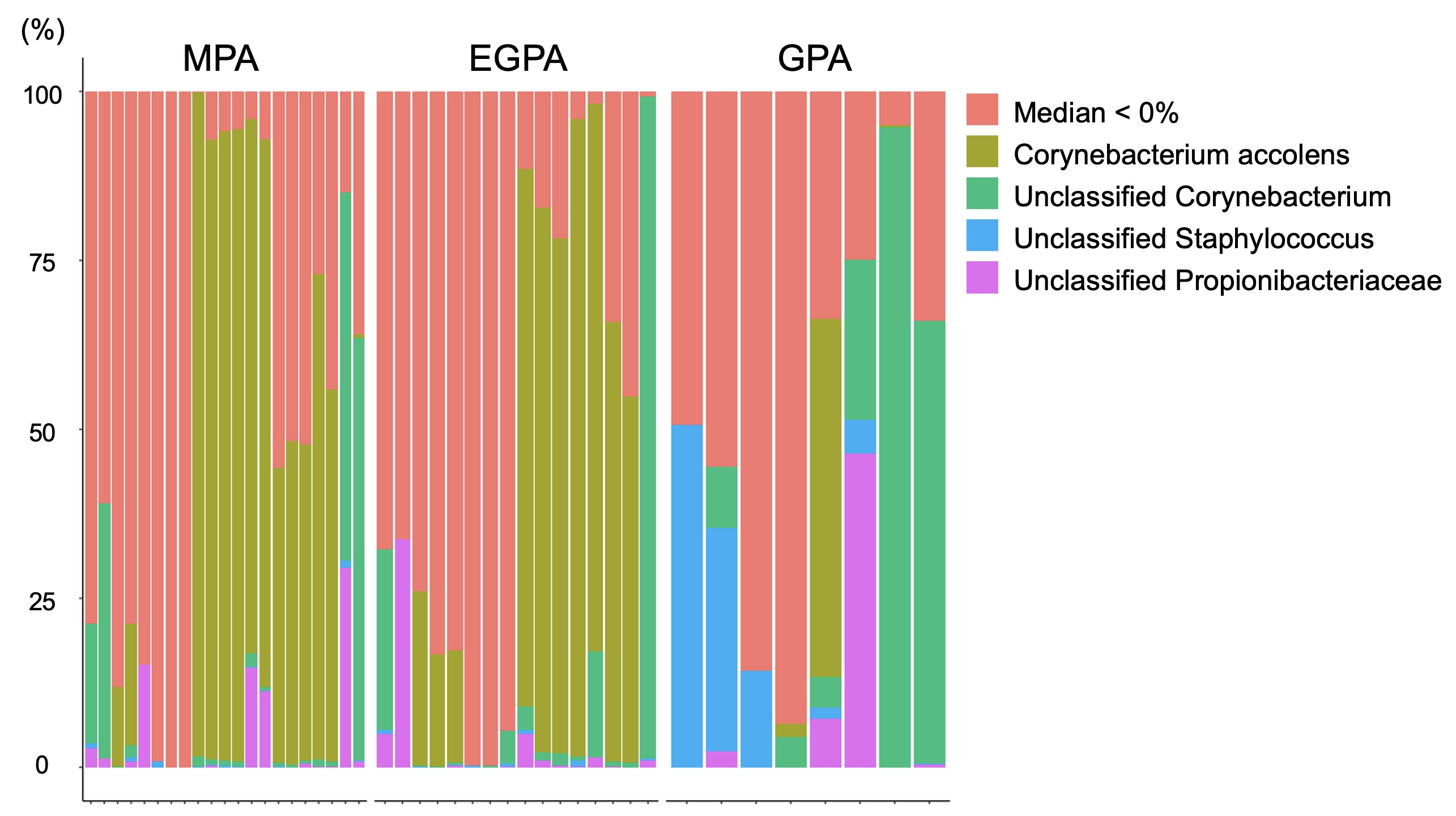
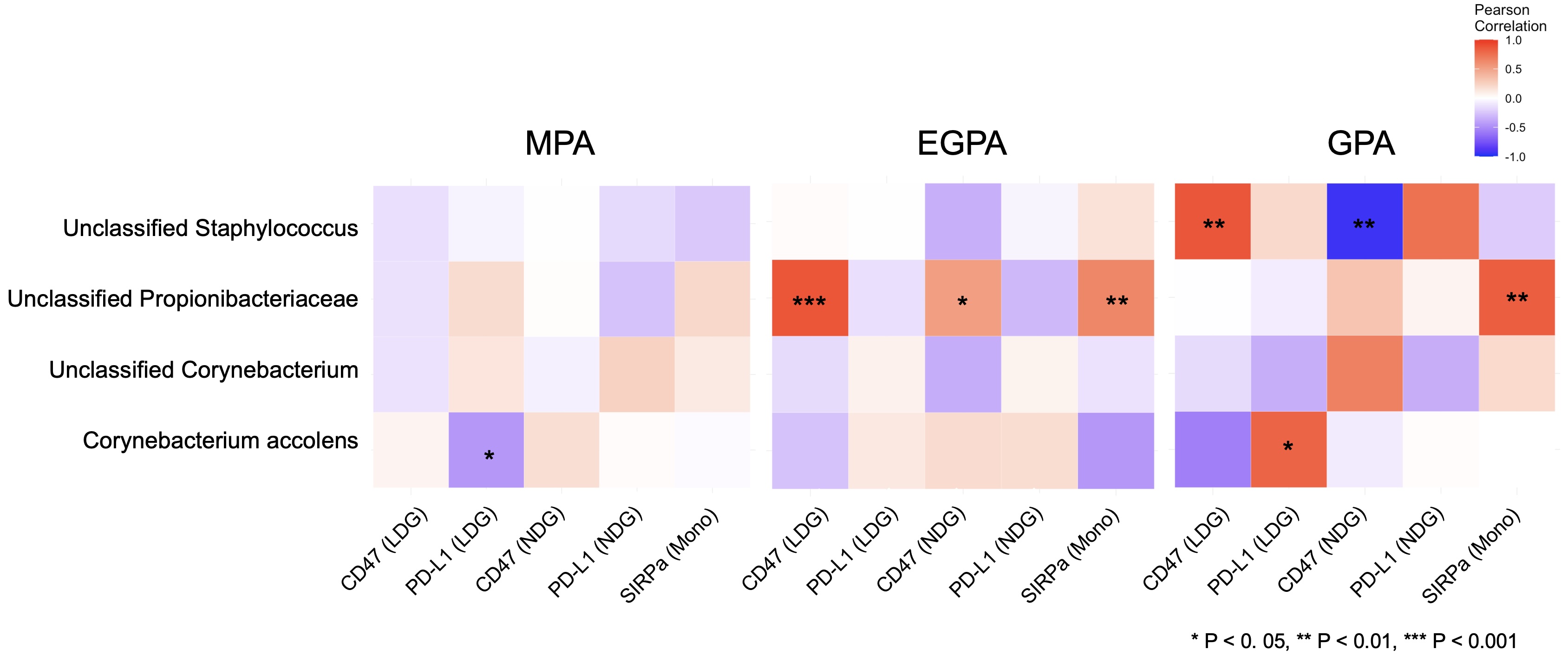
Results: A total of 45 AAV patients were enrolled in the study (8 GPA, 16 EGPA, and 21 MPA). Demographic characteristics of patients are shown in Table 1. The bar plot of relative abundances of species is shown in Figure 1. The heatmap of correlations between nasal taxa and immune checkpoint molecule expressions is shown in Figure 2. Positive correlations between unclassified Propionibacteriaceae and CD47 of NDG and LDG, and SIRPa of monocytes were found in EGPA. CD47 of LDG showed a positive correlation with unclassified Stahpylococcus in GPA, while CD47 of NDG showed the opposite result. Positive correlation between unclassified Propionibacteriaceae and SIRPa of monocytes were found in GPA. Positive correlation between Corynebacterium accolens and PD-L1 of LDG, but not of NDG, was found in GPA. Unclassified Propionibacteriaceae was identified as Propionibacterium acnes and unclassified Staphylococcus was identified as Staphylococcus pettenkoferi using the Basic Local Alignment Search Tool (BLAST).
Conclusion: GPA showed distinct nasal microbiome components compared to MPA and EGPA. GPA and EGPA were suggested to be associated with the expression of immune checkpoint molecules on immune cells in relation to certain specific nasal bacteria, whereas no significant results were obtained for MPA. These results suggest that interactions between the nasal microbiome and host immune systems may differ in each AAV.
To cite this abstract in AMA style:
Nakayama Y, Shirakashi M, Furukawa E, Endo C, Sasai T, Tabuchi Y, Hiwa R, Tsuji H, Kitagori K, Akizuki S, Nakashima R, Murakami K, Yoshifuji H, Morinobu A. Associations Between Immune Checkpoint Molecules and Nasal Microbiome in ANCA-associated Vasculitis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/associations-between-immune-checkpoint-molecules-and-nasal-microbiome-in-anca-associated-vasculitis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/associations-between-immune-checkpoint-molecules-and-nasal-microbiome-in-anca-associated-vasculitis/