Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: ACPA+ individuals without inflammatory arthritis are considered as being in an at-risk state of RA, although further factors are needed to identify individuals with imminent clinical arthritis. Our group has previously demonstrated that HLA-DR+ peripheral helper T cells, which expressed high levels of programmed cell death 1 (PD-1) and T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT), were expanded in the blood from ACPA+ at-risk individuals (ARI), suggesting ongoing T cell dependent immune activation process. Since soluble forms of immune checkpoint proteins (sICPs) were reported to be released from activated T cells, we aimed to investigate the levels of sICPs and chemokines in the serum, and their association with ACPA positivity and development of RA.
Methods: ACPA+ (CCP2, Abbott) ARI without inflammatory arthritis were identified at our university clinic or by antibody screening of volunteers (n = 40). Newly diagnosed ACPA+ RA patients (n = 24), ACPA− RA patients (n = 22) and ACPA− healthy controls (HC, n = 33) were also enrolled. Serum was collected at baseline and ARI were clinically followed. Progression to RA was defined as development of clinical arthritis, fulfilling 2010 ACR/EULAR classification criteria for RA. Using stored serum, we measured 12 molecules including 10 sICPs and 2 chemokines. Associations of these molecules with ACPA positivity, diagnosis of RA, and development of RA were analyzed. ACPA and RF titers were categorized as negative, low, or high using cut-off values by the manufactures’ protocol and the values 3 times higher than upper limit of the cut-off. Cut-off values for sICPs were determined by ROC curves and survival time analysis was performed.
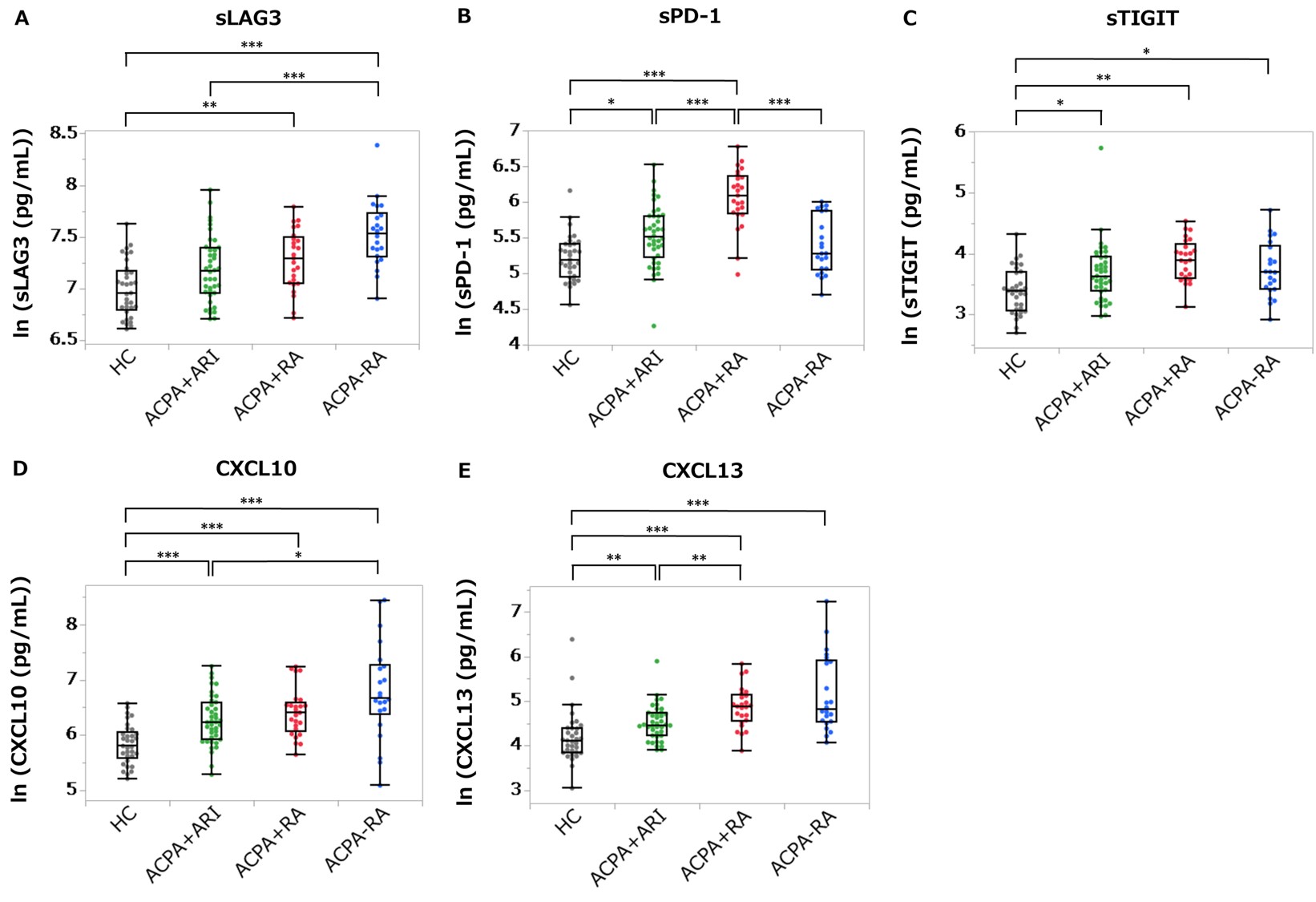
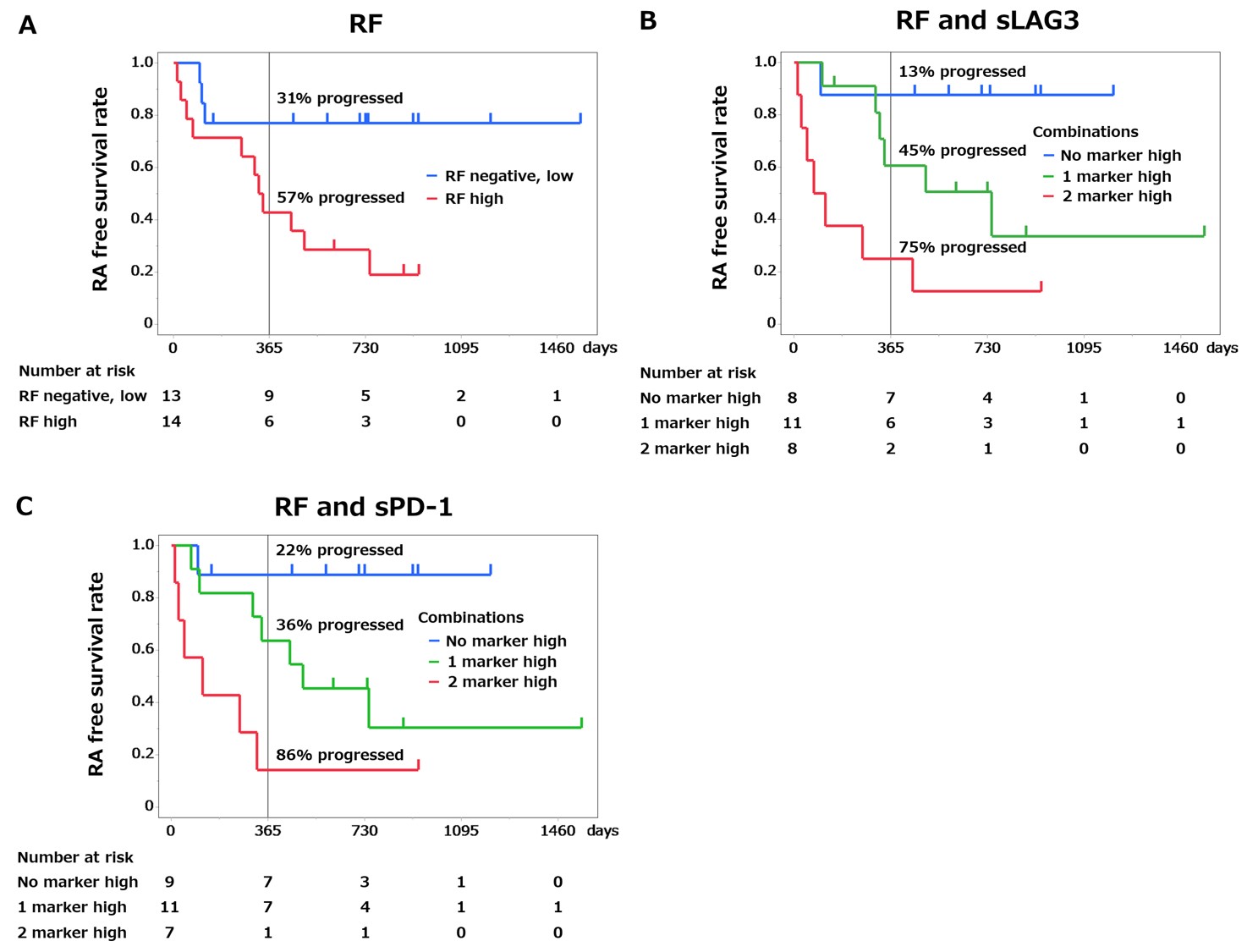
Results: Of 40 ARI, 15 (38%) developed RA during follow-up (median duration 244 days) and 14/15 progressors had high ACPA positivity. Among 12 molecules, soluble PD-1 (sPD-1), soluble TIGIT (sTIGIT), CXCL10 and CXCL13 were significantly elevated in ARI and ACPA+ RA compared to HC. Soluble lymphocyte-activation gene 3 (sLAG3) was significantly elevated in ACPA+ RA and numerically elevated in ARI compared to HC (Figure 1). Following stratification by ACPA titer, we performed survival time analysis in ARI by the combination of RF and one of the molecules (Figure 2). Only sLAG3 and sPD-1 increased hazard ratio (HR) by the combination with RF. Among high ACPA+ ARI (n = 27), HR (95% CI) of developing RA was 7.53 (1.69 to 33.6) in the RFhigh group versus RF− and low group, and 19.7 (2.41 to 161) in the RFhigh sLAG3high group versus RF− and low sLAG3low group, and 19.1 (2.26 to 161) in the RFhigh sPD-1high group versus RF− and low sPD-1low group. Additionally, higher proportions of ARI progressed to RA at one year in the RFhigh sLAG3high group and the RFhigh sPD-1high group than in only RFhigh group (75%, 86% and 57% respectively).
Conclusion: Elevation of sLAG3 or sPD-1 in combination with RF was the risk of imminent development of RA in ARI with high ACPA positivity. This needs to be further validated but elevation of these 2 sICPs in addition to ACPA and RF could be an indicator of a subpopulation of ACPA+ ARI who are at high-risk for imminent RA.
Concentrations of sLAG3 (A), sPD_1 (B), sTIGIT (C), CXCL10 (D) and CXCL13 (E) measured by Meso Scale Discovery assay in the stored serum from ACPA+ at-risk individuals (ARI, n = 40), ACPA+ RA patients (n = 24), ACPA− RA patients (n = 22), and healthy controls (HC, n = 33) are indicated. *P < 0.05, **P < 0.01, ***P < 0.001 by Kruskal-Wallis test and Steel-Dwass test. Lines in each box plot show the median value and top and bottom edges of each box show the third and first quartiles. Top and bottom edges of each whiskers represent the maximum and minimum values other than outliers.
High ACPA+ ARI (n=27) were subdivided according to RF, sLAG3 and sPD_1 status. RA-free survival time analysis according to the baseline serum concentrations by only RF (A), the combination of RF and sLAG3 (B) and the combination of RF and sPD_1(C) are shown. Vertical lines indicate 12 months of follow-up. Proportions of individuals who progressed to RA at one year in each group are indicated.
To cite this abstract in AMA style:
Motoyama R, Nakamura S, Inoue E, Takada H, Harigai M, okamoto Y. Association of Soluble Immune Checkpoint Proteins with the Risk of Developing RA in ACPA-positive At-risk Individuals [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/association-of-soluble-immune-checkpoint-proteins-with-the-risk-of-developing-ra-in-acpa-positive-at-risk-individuals/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-of-soluble-immune-checkpoint-proteins-with-the-risk-of-developing-ra-in-acpa-positive-at-risk-individuals/