Session Information
Date: Monday, November 8, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Outcomes (1257–1303)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: The SLE Responder Index (SRI) is a composite responder definition employed as a clinical trial endpoint in SLE. Despite its widespread adoption, recent discrepant trial results have questioned its performance as an efficacy endpoint, and there is limited validation of the SRI against long-term clinical outcomes. The aim of this study was to investigate longitudinal associations of SRI attainment with key clinical outcomes.
Methods: We used data from a large multi-centre longitudinal SLE cohort. Eligible patients had active disease (SLEDAI-2K ≥6) at a visit designated as baseline. Attainment of a modified version of the SLE Responder Index (mSRI; excluding BILAG criteria) defined as a reduction in SLEDAI-2k ≥4 with no worsening in physician global assessment (PGA) ≥0.3 was determined at 1-year intervals from baseline (up to 5 years). The associations between mSRI attainment and a range of clinical outcomes (longitudinal disease activity, steroid use, flare, damage accrual, attainment of lupus low disease activity state (LLDAS (Golder 2019)), clinical remission on therapy (CROT (van Vollenhoven 2017)) and mortality) were determined using univariable linear and logistic regression.
Results: A total of 2,024 patients had an eligible baseline visit. Baseline characteristics are summarised in Table 1. mSRI response was attained by 55.5% of patients at 1 year, with similar rates (54-57%) at subsequent annual visits. Associations of mSRI response with clinical outcomes were similar at each of the annual time points; Table 2 presents results at years 1, 3 and 5 post baseline. Attainment of mSRI was associated with significantly lower disease activity over follow-up. Damage accrued in 9% of patients at 1 year and 32% by 5 years, with rates numerically lower in mSRI responders at all time points (significant at years 2 and 3). mSRI response was also significantly associated with attainment of LLDAS and clinical remission on therapy. Prednisolone doses at follow-up visits were consistently lower in the mSRI responder group, but time-adjusted mean dose had no significant differences. mSRI response was not associated with significant protection against flare. There were insufficient deaths for meaningful between-group comparison.
Conclusion: In a longitudinal cohort of SLE patients with baseline SLEDAI ≥6, attainment of a modified SRI responder index at annual visits up to 5 years was associated with clinical benefit including lower disease activity, reduced damage accrual and higher attainment of treat-to-target endpoints.
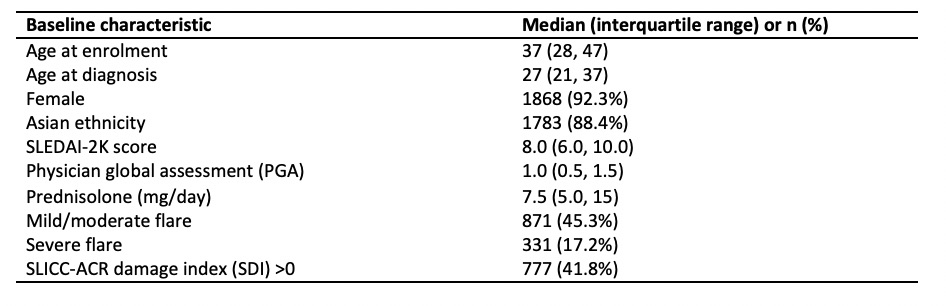 Table 1: Baseline characteristics (n=2024)
Table 1: Baseline characteristics (n=2024)
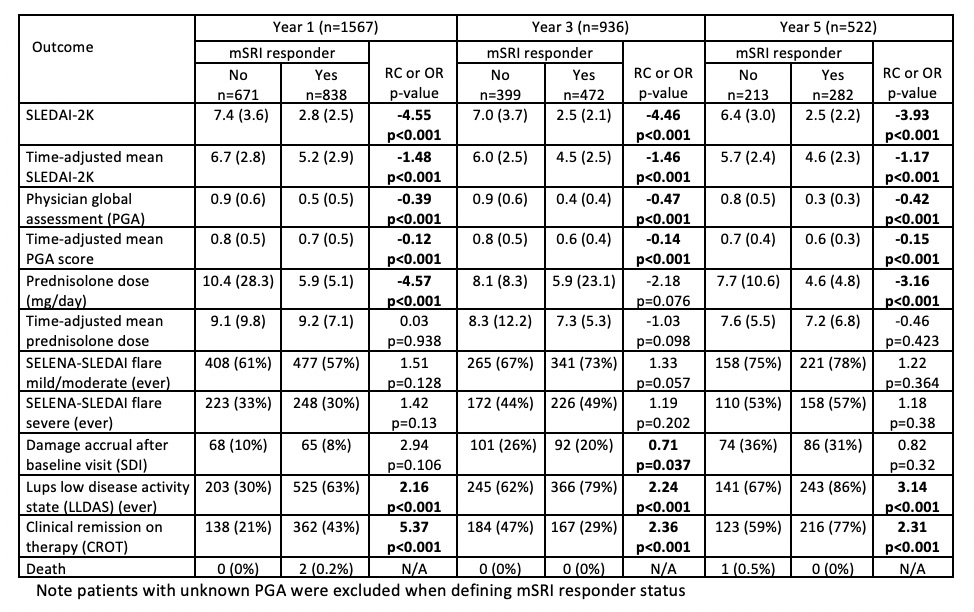 Table 2: Associations between mSRI attainment and clinical outcomes at follow up years 1, 3 and 5
Table 2: Associations between mSRI attainment and clinical outcomes at follow up years 1, 3 and 5
To cite this abstract in AMA style:
Connelly K, Kandane-Rathnayake R, Hoi A, Louthrenoo W, Hamijoyo L, Cho J, Lateef A, Luo S, Wu Y, Lau C, CHEN Y, Navarra S, Zamora L, Li Z, An Y, Sockalingam S, Hao Y, Zhang Z, Chan M, Katsumata Y, Harigai M, Oon S, Bae S, O’Neill S, Gibson K, Kikuchi J, Basnayake B, Takeuchi T, Ng K, Goldblatt F, Law A, Tugnet N, Tanaka Y, Kumar S, Tee M, Tan J, Karyekar C, Nikpour M, Golder V, Morand E. Association of SLE Responder Index (SRI) Attainment and Long-term Clinical Outcomes [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/association-of-sle-responder-index-sri-attainment-and-long-term-clinical-outcomes/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-of-sle-responder-index-sri-attainment-and-long-term-clinical-outcomes/
